Answers
Answer:
0.000237mL
Explanation:
0.237 x 10^-6L = 0.000000237L = mL
0.000237mL
Related Questions
Which option gives an object's volume in SF units?
O
A. 4.3 kg
B. 2.6 m3
C. 5.5 K
D, 3.4L
SUBMIT
Answers
Answer:
The correct answer is:
2.6 m3 (B)
Explanation:
SF unit stands for Stowage factor unit. Stowage factor is the volume occupied by one unit of mass (weight) when stowed in cargo space. SF expresses the unit in cubic meters (m³) or cubic feet. SF is a unit of measurement that indicates how much space (volume) a particular unit of cargo occupies in a ship's cargo compartment
Explain the scientific meaning and use of the word "transformation".
Answers
Transformation in chemistry is scientifically used to explain the process of changing one compound to another in a chemical reaction.
What is transformation?The word "transformation" has a very special significance in chemistry. We know that in English, to transform would simply imply to change from one form to another. This is not quite far from its meaning in the parlance of chemistry.
The word transformation is normally applied in the area of chemical reactions especially as it has to do with reaction with in organic chemistry. It has to do with the change from one molecule to another and this is of great importance in the discussion of synthetic chemistry.
As such, the word transformation in chemistry is scientifically used to explain the process of changing one compound to another in a chemical reaction.
Learn more about chemical transformation:https://brainly.com/question/8210521
#SPJ1
Compare the 13C spectra of starting material and product and explain which peaks can be used to unambiguously determine whether your oxidation was successful. -g
Answers
Answer:
The description of the given question is summarized in the explanation section below.
Explanation:
A maximum of around 82 ppm would be found throughout the beginning material spectrum around CDCl3. This same resulting spectrum maximum of 82 ppm has been lost, as well as the section has a historic high of 215 ppm.
The OH summit at 82 ppm was obvious as well as a significant maximum of around 215 ppm carboxylic acid was observed. That justifies the occurrence of oxidizing, C13 NMR was indeed verified as a reactive organic compound oxidized in carbonyl Ozone.The oxidation state is successful by comparing the 13C spectra of starting material and product.
What is 13C spectra?The 13 carbon spectroscopy also known as 13C NMR is the application of NMR to carbon atom.
A frequent task in synthetic reactions involving rearrangement, conversion, or cyclization is to compare the NMR spectra of the starting material and the result.
Around CDCl3, a high of around 82 ppm can be detected throughout the starting material spectrum.
The section's historic high of 215 ppm has also been destroyed, as has the resulting spectrum maximum of 82 ppm.
The OH peak at 82 ppm was clearly visible, as was a substantial maximum at around 215 ppm carboxylic acid.
Thus, It can say that the oxidation state was successful.
Learn more about 13C spectra, here:
https://brainly.com/question/6427502
a metal worker used a cutting torch that operated by reacting acetylene gas with oxygen gas, as shown in the unbalanced equation below. balance the following equation for the reaction of acetylene and oxygen, using the smallest whole-number coefficients. (the values are 1,2,3,4,5)

Answers
The balanced equation for the reaction of acetylene (C₂H₂) and oxygen (O₂) is; 2 C₂H₂(g) + 5 O₂(g) → 4 CO₂(g) + 2 H₂O(g) + heat
The coefficients in the balanced equation represent the stoichiometric ratio of the reactants and products in the chemical reaction. In this case, 2 molecules of acetylene (C₂H₂) react with 5 molecules of oxygen (O₂) to produce 4 molecules of carbon dioxide (CO₂) and 2 molecules of water (H₂O), along with the release of heat.
The balanced equation shows that the number of atoms of each element is the same on both the sides of the equation, in accordance with the law of conservation of mass.
To know more about acetylene here
https://brainly.com/question/20529866
#SPJ1
A template of a Venn diagram representing common and differentiating characteristics of covalent and ionic bonds is shown. Which of the following characteristics can be written only in space C?
Answers
Covalent and ionic bonds refer to atoms joined by their electrons. In covalent bonds, electrons are shared by the involved non-metal atoms. Option 2 is correct. Occurs due to the sharing of electrons between two non-metal atoms.
What are covalent and ionic bonds?
Both of them, covalent and ionic bonds, are chemical bonds that can form between atoms.
Ionic bonds occur between atoms with different electronegativity. When they bind, they transfer electrons from one atom to the other creating ions with opposite charges that attract each other.
Ionic compounds are formed by anions and cations.
• Cations are positive ions derivated from metals.
• Anions are negative ions derivated from non-metals.
The metal atoms share its electrons with the non-metal ones, creating stable configurations. Ionic bonds do not create molecules.
Covalent bonds are formed between atoms share electrons to be more stable. Atoms involved share electrons equally, creating a strong bond between them.
Covalent bonds are usually formed between non-metal atoms.
Option 2 is correct. Occurs due to the sharing of electrons between two non-metal atoms
You can learn more about covalent and ionic bonds at
https://brainly.com/question/19739192
#SPJ1
Complete question
A template of a Venn diagram representing common and differentiating characteristics of covalent and ionic bonds is shown.
Which of the following characteristics can be written only in space C?
On the diagram,
The non-overlapping space on the left is marked A, and belongs to the IONIC BOND side of the diagram.The overlapping space is marked B The non-overlapping space on the right is marked C, and belongs to the COVALENT BOND side of the diagram.Options,
Formed between positively and negatively charged ionsOccurs due to the sharing of electrons between two non-metal atomsOccurs in substances that are mostly solids at normal temperature and pressureFormed between an atom with very high electronegativity and an atom with very low electronegativityDetermine the heat (q) required to raise the temperature of 80.0 grams of metal from 28.0 °C to 53.0 °C. Assume the specific heat of the material is 0.8 J/g°C. Be sure to use proper labels, fill in all blanks, and use complete sentences where applicable for full credit.
Heat (q) = ______________ J
Mass (m) = ____________ g
heat (s) = ______ J/g°C
Initial temp (T1) = ______ °C
Final temp (T2) = _______ °C
Is the process endothermic or exothermic? _______________________________
Answers
Answer:
Explanation:
m= 80.0 g
Cp=0.8 J/gc
Change in T= 25
q=Cp X m X T =0.8 X 80.0 X 25 =1600J
Postive q or enthalpy makes it endothermic.
What is the pH of 6.00 M H2CO3 if it has 7% dissociation? SHOW YOUR WORK!!!
Answers
From the calculation, the pH of the solution can be obtained as 0.39.
What is the pH of the solution?We first have to obtain the dissociation constant of the solution;
α = √Ka/C
Ka = Cα^2
Ka = 4.9 * 10^-3 * 6
Ka = 0.0294
Then we have that;
0.0294 = [x]^2/[6 - x]
0.0294 * [6 - x] = x^2
0.1764 - 0.0294 x = x^2
x^2 + 0.0294 x - 0.1764 =0
x = 0.41 M
Thus the pH of the solution is;
pH = -log[0.41]
= 0.39
pH is an important parameter in chemistry, biology, and many other fields, as it can affect the behavior and properties of substances and reactions.
Learn more about pH:https://brainly.com/question/15289714
#SPJ1
What is the mass of 5.6 x 1026 molecules of Al2O3?
Answers
Answer:
\(94,883.72\text{ g}\)Explanation:
Here, we want to get the mass that contains the given number of molecules
We start by getting the number of moles
Mathematically:
\(1\text{ mole has 6.02 }\times\text{ 10}^{23}\text{ molecules}\)The number of moles in the given number of molecules would be:
\(\frac{5.6\text{ }\times\text{ 10}^{26}}{6.02\text{ }\times\text{ 10}^{23}}\text{ = 930.23 moles}\)Now from here, we get the mass
Mathematically:
\(mass\text{ = number of moles }\times\text{ molar mass}\)The molar mass of the compound is 102 g/mol
Thus, we have the mass as:
\(930.23\text{ }\times\text{ 102 = 94,883.72 g}\)What kinds of matter have thermal energy
Answers
Answer:
Thermal energy comes from heat sources that provide energy from it's temp.
Explanation:
Produced when a rise in temperature causes atoms and molecules to move faster and collide with each other. The energy that comes from the temperature of the heated substance is called thermal energy. 6 min, 47 sec Heat Energy.
(If this doesn't help let me know in the comments, I'll try to explain better :> )
What quantity of heat (in kJ) will be absorbed by a 92.7 g piece of aluminum (specific heat = 0.930 J/g・ °C) as it changes temperature from 23.0 °C to 67.0 °C?
Answers
The quantity of heat absorbed by the aluminum is 3.1257 kJ.
What is heat?
Heat is a form of energy that is transferred between two objects as a result of a difference in temperature. It flows from an object at a higher temperature to one at a lower temperature until both objects reach thermal equilibrium, i.e., they have the same temperature. Heat can be transferred through conduction, convection, and radiation.
To calculate the heat absorbed by a piece of aluminum, we can use the following formula:
q = m * c * ΔT
where q is the heat absorbed, m is the mass of the aluminum, c is the specific heat of aluminum, and ΔT is the change in temperature.
Substituting the given values, we get:
q = 92.7 g * 0.930 J/g・ °C * (67.0 °C - 23.0 °C)
q = 3,125.7 J or 3.1257 kJ (to four significant figures)
Therefore, the quantity of heat absorbed by the aluminum is 3.1257 kJ.
What is radiation?
Radiation refers to the emission or transmission of energy through space or a medium in the form of waves or particles. This can include electromagnetic radiation such as visible light, radio waves, and X-rays, as well as particle radiation such as alpha and beta particles. Radiation can occur naturally, such as from the sun or from radioactive elements in the earth's crust, or it can be produced artificially, such as in medical imaging or nuclear power generation. Depending on the type and intensity of radiation, it can have various effects on living organisms and materials.
To know more about heat, visit:
https://brainly.com/question/12911869
#SPJ1
Complete question is: 3.1257 kJ quantity of heat (in kJ) will be absorbed by a 92.7 g piece of aluminum (specific heat = 0.930 J/g・ °C) as it changes temperature from 23.0 °C to 67.0 °C.
Question 1 (5 points)
Calculate the frequency of a photon that exhibits a wavelength of 451 nm.
Equation: E = hu
h = 6.63 x 10-34]'s
Answers
Answer:
e
Explanation:
describe an example of temperature causing a change in the size of matter.
Answers
Change in temperature when quicklime reacts with water.
What's an example of a temperature change?When ice cubes are separated from the freezer and conducted to room temperature, they turn into a liquid. This temperature is the boiling point of water. Heat makes water molecules move fast. Cooling air causes water vapor to change to a liquid. Water changes rapidly into a gas when water is heated to a temperature of 100 degrees Celsius or 212 degrees Fahrenheit.
When energy is free in an exothermic reaction, the temperature of the reaction mixture increases. When energy is soal up in an endothermic reaction, the temperature decreases.
So we can conclude that Some chemical reactions are distinguished by changes in temperature.
Learn more about temperature here: https://brainly.com/question/25677592
#SPJ1
why does lead exist in a higher amount in brown algae than plankton?
Answers
Lead levels in plankton and algae are high, mostly as a result of environmental pollution brought on by human activity. While it is true that some brown algae species have the ability to accumulate heavy metals like lead.
Plankton and algae have high levels of lead, mostly as a result of environmental contamination brought on by human activities including mining, industrial operations, and the burning of fossil fuels.
Due to the fact that plankton and algae take up trace quantities of lead from the surrounding water, their tissues contain greater concentrations of the metal.
Learn more about brown algae, here:
https://brainly.com/question/31714795
#SPJ1
lphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
Which substance has Delta.Hf defined as 0 kJ/mol?
A. H2O (s)
B. Ne (l)
C. F2 (g)
D. CO2 (g)
Answers
Answer:
its actually c
Explanation:
1. Which of the following is a Cationic single replacement reaction
a) 2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
b) C3H60 + 4 02 → 3 CO2 + 3 H2O
c) SeClo + O2 → SeO2 + 3Cl2
d) 2 NO2 → 2 O2 + N2
Answers
Answer:
I believe the answer for this is A
Which types of particles are charged and located within the nucleus of an atom?
a. protons and electrons
b. neutrons
c. electrons
d. protons
Answers
Which statement about erosion is true? Most erosion occurs too slowly to observe. Most erosion occurs too quickly to observe, Erosion can happen very quickly or very slowly. It is not possible to measure erosion rates.
Answers
Answer:
Erosion can happen very quickly or very slowly.
e.g splash erosion occurs slowly while gulley erosion occurs very fast.
Identify the type of reaction. Complete the equations with the correct reactants then balance each equation.

Answers
The balanced equations are Cd (g) + S\(_8\) (g) → CdS (g), K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\). and a Na + HOH → NaOH + H\(_2\). Cd (g) + S\(_8\) (g) → CdS (g) is combination reaction.
An equation per a chemical reaction is said to be balanced if both the reactants plus the products have the same number of atoms and total charge for each component of the reaction. In other words, both sides between the reaction have an equal balance of mass and charge. The balanced equations are Cd (g) + S\(_8\) (g) → CdS (g), K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\). and a Na + HOH → NaOH + H\(_2\).
Cd (g) + S\(_8\) (g) → CdS (g) = combination reaction
K\(_2\)CO\(_3\) → K\(_2\)O + CO\(_2\) = decomposition reaction
Na + HOH → NaOH + H\(_2\) = Hydration reaction
To know more about balanced equation, here:
https://brainly.com/question/7181548
#SPJ1
What is the chemical formula for micas
Answers
Answer:
X2Y4–6Z8O20 (OH, F)4
Explanation:
The chemical formula for micas is X2Y4–6Z8O20 (OH, F)4, where X is K, Na, or Ca or less commonly Ba, Rb, or Cs; Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.; Z is chiefly Si or Al, but also may include Fe3+ or Ti1. Structurally, micas can be classed as dioctahedral (Y = 4) and trioctahedral (Y = 6)1.
The chemical formula for micas varies, but they typically have the general formula:
(K,Na,Ba,Rb,Ca)(Al,Mg,Fe)2(Si3Al)O10(OH,F)2
Where:
K, Na, Ba, Rb, and Ca represent alkali metals and alkaline earth metals that can occupy the interlayer sites. Potassium is the most common.Al and Mg represent aluminum and magnesium that occupy the octahedral sites between the silica tetrahedral sheets.Fe can substitute for Al in the octahedral sites.Si and Al occupy the tetrahedral sites within the silica sheets. The ratio of Si to Al is typically around 3:1.O represents oxygen atomsOH or F can occupy the interlayer sites, with hydroxyl (OH) being more common. Fluorine can substitute for hydroxyl in some micas.So in summary, micas have a layered aluminosilicate structure with interlayer cations that can vary, but they are generally characterized by an approximate 3:1 ratio of silicon to aluminum within the silica tetrahedral sheets. The chemical formula given is the generalized structural formula for micas, but the actual compositions can vary based on the specific mica.
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
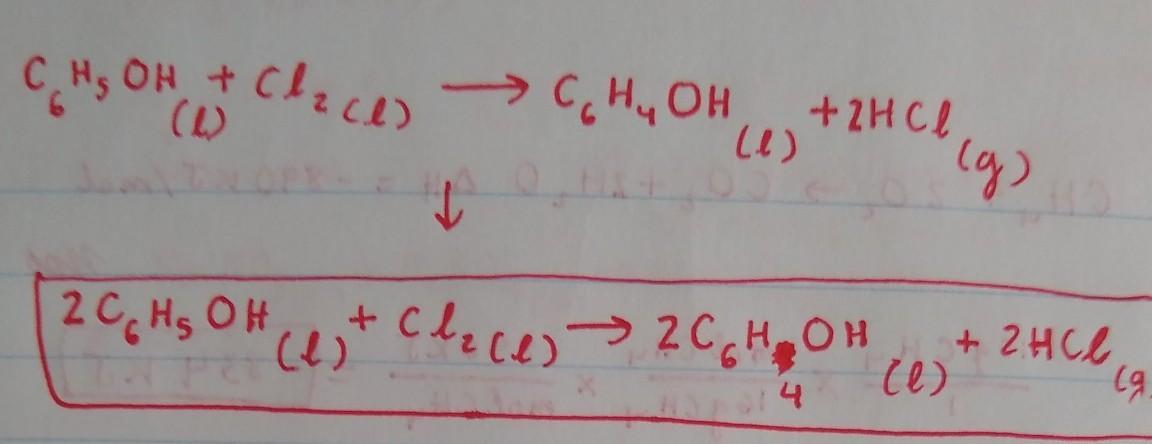
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
In class we derived the Gibbs energy of mixing for a binary mixture of perfect gases. We also discussed that the same result is obtained for liquids when the resulting solution is ideal. For real solutions we introduced activities and activity coefficients. Derive the molar Gibbs energy of mixing and the molar excess Gibbs energy of mixing in terms of activity coefficients.
Answers
Answer:
Attached below
Explanation:
Free energy of mixing = ΔGmix = Gf - Gi
attached below is the required derivation of the
a) Molar Gibbs energy of mixing
ΔGmix = Gf - Gi
hence : ΔGmix = ∩RT ( X1 In X1 + X2 In X2 + X3 In X3 + ------- )
b) molar excess Gibbs energy of mixing
Ni = chemical potential of gas
fi = Fugacity
N°i = Chemical potential of gas when Fugacity = 1
ΔG = RT In ( a2 / a1 )

You breed poodles and notice there is bimodal distribution of poodle heights in your kennel. One group, with contains 24 dogs, averages 20.5 inches, another group that contains 12 dogs, averages 32.1 inches. What is the average height of the poodle in you kennel?
Answers
The average height of the poodle in your kennel is 1.46.
What is the average?The term average has to do with the mean value of something now we have been told that one group, with contains 24 dogs, averages 20.5 inches, another group that contains 12 dogs, averages 32.1 inches.
The first group has 24 dogs and the second group has 12 dogs the total number of dogs is 24 + 12 = 36 dogs. The total height of the dogs is 20.5 inches + 32.1 inches = 52.6 inches
The average height of the poodle in your kennel = 52.6 inches/36 dogs
= 1.46
Learn more about average height:https://brainly.com/question/14526422
#SPJ1
What is the freezing point in C of a 0.520 m aqueous solution of NaCl?

Answers
The freezing point of 0.520M aqueous solution of NaCl is -0.97°C.
How to calculate freezing point?Freezing point is the temperature at which a liquid freezes, and the solid and liquid phases are in equilibrium; it is normally the same as the melting point.
To calculate the freezing point of a solution, we can use the following formula;
∆T = K m
Where;
K = freezing point constantm = molalityAccording to this question, the molality of a solution is 0.52m.
∆T = 1.86°C/m × 0.52m
∆T = 0.967°C
Freezing point = 0.0°C - 0.97°C
Freezing point = -0.97°C
Therefore, -0.97°C is the freezing point of the solution.
Learn more about freezing point at: https://brainly.com/question/30168966
#SPJ1
Answer:
-1.934
Explanation:
0 - {(0.520) x (1.86) x (2)}
4. A handful of sand has a mass of 208 g and displaces a volume of 80.0 mL. Calculate its
density (g/mL).
28
Answers
Answer:
2.6 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{208}{80} = 2.6 \\ \)
We have the final answer as
2.6 g/mLHope this helps you
Answer these questions. What are metals? Write the position of alkali metals and alkaline earth metals in the modern periodic table.
Answers
Explanation:
a metal is an element that readily forms positive ions (cations) and has metallic bonds.
Group two of the periodic table comprises the elements beryllium, magnesium, calcium, strontium, barium and radium. The elements in this group, which are all shiny and silvery-white in appearance, are known as the alkaline earth metals. Like the alkali metals, they form alkaline solutions when they react with water.
A student mixes baking soda with acetic acid in a test tube. The student observes the formation of a gas and records the two reactions that occur.
The student concludes that the two reactions follows the Law of Conservation of Matter. Which statement supports the student's conclusion?

Answers
The number of atoms in the reactants and products of each reaction is the same. The last option.
Law of conservation of matterThe law of conservation of matter states that during chemical reactions, matter can neither be created nor destroyed but can be converted from one form to another. This law is also applicable to energy and atoms during chemical reactions.
In other words, all chemical reactions, as a rule of thumb, obey the law of conservation. Thus, for any chemical reaction:
Energy is conservedMatter is conservedThe number of atoms of different elements is conservedTherefore, the statement that supports the student's conclusion that the two reactions follows the law of conservation of matter would be that the number of atoms in the reactants and products of each reaction is the same.
More on the law of conservation can be found here: https://brainly.com/question/20635180
#SPJ1
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol.
Answers
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol, the molecular formula is NO₂.
We must compute the empirical formula in order to ascertain the compound's chemical composition.
If we have 100 grams of the compound.
This suggests we have:
30.4 g of nitrogen
69.6 g of oxygen
Now, we have to convert the mass of each element to moles.
The molar mass of nitrogen (N) = 14.01 g/mol
the molar mass of oxygen (O) = 16.00 g/mol.
Number of moles of nitrogen (N):
2.17 mol
Number of moles of oxygen (O):
4.35 mol
The simplest whole-number ratio between the moles of nitrogen and oxygen must now be determined. To calculate the ratio, we divide both numbers by the smaller value.
Moles N / moles O = 2.17 mol / 2.17 mol = 1.00
Moles O / moles O = 4.35 mol / 2.17 mol = 2.00
The ratio is approximately N₁O₂.
We divide the subscripts by their greatest common divisor to obtain the simplest ratio, since we are looking for the empirical formula. The empirical formula is NO₂ since the greatest common divisor in this situation is 1.
The molecular formula of the compound is NO₂.
Learn more about molecular formula, here:
https://brainly.com/question/11203434
#SPJ1
What subatomic particle is not considered when calculating atomic
mass?
electrons
protons
neutrons
Answers
Answer:
Electrons are not considered.
Explanation:
An electron's mass is around 9.1 * 10^-31 kg, whereas the mass of protons and neutrons is around 1.7 * 10^-27 kg. Since electrons are about 1000x smaller than protons and neutrons, they are not considered to contribute significantly to the atomic mass.