Answers
Answer:The suggested answers are for K=298 degrees and the nearest correct answer seems to be increase the room temperature by 22 degrees Fahrenheit. But by calculation, for 300 K, then convert 300k to degrees Celsius = 300-273.15=26.85 degrees celsius. Then convert the 26.85 to degrees F, so F=9/5C + 32= 48.33+32=80.33-55F (present room temperature)=25.33 degrees F to increase the room temperature by.
Explanation:
According to conversion between Celsius and kelvin temperatures , 363.6 K is 90.6°C.
What is the conversion between kelvin and Celsius scales?Three scales are used to measure temperature the Kelvin scale, Celsius scale and Fahrenheit scale.The basic unit of temperature which is used in SI system is kelvin temperature however it is impractical in most practical uses.
The Celsius scale is commonly used in clinical terms where it is related to kelvin scale as the two units are equal in magnitude. The difference between Celsius and kelvin scale is the zero point which on kelvin scale represents theoretical temperature at which substance has no further heat loss.
While the zero point on Celsius scale represents freezing point of water. The two scales are related as follows
K=°C+273
Learn more about conversion between Celsius and kelvin,here:
https://brainly.com/question/3168451
#SPJ2
Related Questions
100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch
Calculate the g and mL necessary to make this solution
Answers
52.6g and 23.8mL necessary 100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch to make this solution.
What is malonic acid?
The chemical formula of malonic acid is CH2(COOH)2. Malonates include the ionized form of malonic acid as well as its esters and salts. Because it interferes with respiration, malonic acid is extremely harmful, especially in cases of cancer and other degenerative disorders (the making of ATP in mitochondria). Malonic acid is a somewhat unstable substance with limited practical uses. Beetroot contains its calcium salt, however the acid itself is often made by hydrolyzing diethyl malonate.
To learn more about malonic acid, refer: -
https://brainly.in/question/46046349
SPJ1
A solution contains 0.470 mol of isopropanol (C3H7OH) dissolved in 3.320 mol of water
a)What is the mole fraction of isopropanol?
b)What is the mass percent of isopropanol?
c)What is the molality of isopropanol?
Answers
A. The mole fraction of isopropanol in the solution is 0.124
B. The mass percent of isopropanol is 12.4%
C. The molality of isopropanol is 7.86 M
A. How to determine the mole fraction of isopropanolMole of isopropanol = 0.470 moleMole of water = 3.320 mole Total mole = 0.470 + 3.32 = 3.79 mole Mole fraction of isopropanol =?Mole fraction = mole / total mole
Mole fraction of isopropanol = 0.470 / 3.79
Mole fraction of isopropanol = 0.124
B. How to determine the percentage of isopropanolMole of isopropanol = 0.470 moleTotal mole = 3.79 mole Percentage of isopropanol =?Percentage = (mole / total mole) × 100
Percentage of isopropanol = (0.470 / 3.79) × 100
Percentage of isopropanol = 12.4%
C. How to determine the molality Mole of isopropanol = 0.470 moleMole of water = 3.320 mole Mass of water = 3.320 × 18 = 59.76 g = 59.76 / 1000 = 0.05976 KgMolality of isopropanol =?Molality = mole / Kg of water
Molality of isopropanol = 0.47 / 0.05976
Molality of isopropanol = 7.86 M
Learn more about Molality:
https://brainly.com/question/4251997
In which environment is erosion likely to have the most impact?
a steep hillside with little vegetation and strong downpours
a steep hillside covered with grasses and mild downpours
a flat valley with little vegetation and heavy downpours
a flat valley covered with grasses and mild downpours
Answers
Answer:
A. Steep hillside, little vegetation, strong downpours
Explanation:
between a steep hill and a flat valley, the hill is more likely to be affected because of gravity. with a valley theres less places for the dirt to actually move to. So C and D are not correct
a lack of vegetation and heavy downpour is the perfect condition for erosion. plants' root system makes dirt and rock more stable. without a lot of roots to hold the dirt in place and absorb water before it runs over the rocks and breaks them down, heavy rain would be a lot more likely to erode the area. so B isnt right either
Consider the following reversible reaction.
C(s) + O₂(g) → CO₂(g)
What is the equilibrium constant expression for the given system?
[CO₂]
0 Keq=
[C][0₂]
O
о Keq=
[CO₂]
[0₂]
[C][0₂]
O Kea [CO₂]
[0₂]
O Kea= [CO₂]
Answers
The equilibrium constant expression for the given system is: Keq = [CO₂] / [C][O₂]
What is equilibrium constant?Equilibrium constant, Keq for a given is defined as the concentration of the products raised to their coefficient to the concentration of the reactants raised to their coefficient
For example:
eB <=> cD
The equilibrium constant for the reaction above is given as
Keq = product / reactant
Keq = [D]^c / [B]^e
With the above illustration, we can obtain the equilibrium constant for the reaction given in the question. Details below:
How to determine the equilibrium constant expressionFrom the question given above, the following data were obtained:
Equation: C(s) + O₂(g) → CO₂(g)Equilibrium constant (Keq) =?The equilibrium constant for the reaction reaction can be obtained as follow:
Keq = product / reactant
Keq = [CO₂] / [C][O₂]
Learn more about equilibrium constant:
https://brainly.com/question/17960050
#SPJ1
The process by which vesicles move substances out of a cell is
Answers
Which statement about members of a homologous series is true

Answers
The statement "Each member of a homologous series differs from its nearest neighbors by 14 amu" is true of members about a homologous series.
What are homologous series?In organic chemistry, a homologous series unveils itself as a sequential assembly of compounds exhibiting an identical functional group, boasting akin chemical traits. Within this series, the constituents can either sport a branched or unbranched structure, or deviate through the molecular formula of CH2 and a molecular mass variation of 14u.
This divergence may manifest as the elongation of a carbon chain, as observed in the linear alkanes (paraffins), or as the augmentation in the count of monomers forming a homopolymer, such as amylose.
The entities belonging to a homologous series typically embrace a fixed assortment of functional groups, thereby conferring upon them resemblant chemical and physical characteristics.
Learn about homologous series here https://brainly.com/question/1569487
#SPJ1
Styrofoam has a density of 0.075 g/mL. What is the volume of 21.80 g of Styrofoam?
Answers
The answer is 290.66 mL
Explanation:
The density of any substance including styrofoam is determined by two main factors: mass (atoms in the substance) and volume (space occupied). Moreover, the volume or mass of the substance can be calculated by using the density as these variables are related. Below I show the process:
density = mass / volume
mass = density x volume
volume = mass / density
volume = 21.80 g / 0.075 g/mL
volume = 290.66 mL
Which of the following units would you use to describe width of a small piece of metal? Ounce Mole Millimeter Milliliter
Answers
Answer:
Millimeter can be used to describe the width of a small piece of metal
Explanation:
The most appropriate unit to describe the width of a small piece of metal would be millimeters (mm). Millimeters are commonly used to measure small lengths or dimensions, such as the width of objects. This unit provides a precise and accurate measurement for small-scale items.
Ounces (oz) are typically used to measure weight or volume and may not be suitable for describing the width of an object.
Moles (mol) are used in chemistry to quantify the amount of a substance, so it is not relevant for measuring dimensions.
Milliliters (ml) are used to measure volume, specifically the capacity of liquids, and are not typically used to describe the width of a solid object.
Therefore, when discussing the width of a small piece of metal, millimeters (mm) would be the appropriate unit as it specifically measures small lengths or dimensions.
AI said C. Millimeter.
I hope this helped! :)
Which treatment(s) will help remove contaminants from minerals or from the pipes carrying water from a source? you can select more than one (Water Contamination Gizmos) **ONLY ANSWER IF YOU ACTUALLY KNOW ❗️❗️**
answer choices:
Sedimentation
Disinfection
Filtration
Coagulation

Answers
Sedimentation, filtration, and coagulation are the treatments that will help remove contaminants from minerals or from the pipes carrying water from a source.
Sedimentation is a process in which suspended particles settle out of water. It is one of the most basic techniques for removing particles from water. As particles settle, they become trapped in the bottom of a container or settle to the ground in an outdoor setting
Filtration is a method of removing particles from a fluid. It is a physical or chemical separation method that separates solids from fluids (liquids or gases) by adding a medium through which only the fluid can pass.
Coagulation is the process of using chemicals to remove contaminants from water. By creating a chemical reaction, coagulation destabilizes particles and causes them to clump together. This helps to remove the contaminants from the water.
Disinfection is the process of eliminating or destroying pathogens that cause infection. Disinfection eliminates harmful microorganisms by destroying or inactivating them. The disinfectant is a chemical or physical agent that is used to destroy or inactivate harmful microorganisms.
Know more about Filtration here:
https://brainly.com/question/29756050
#SPJ8
Balance the redox reaction:Cr2O7^2-+C2O4^2---->Cr^3++CO2
Answers
First let's discover which element is oxidizing and which is reducing
So first let's rewrite the equation here:
Cr2O7^2- + C2O4^-2----> Cr^3+ + CO2
Reactant side:
oxidation state for Cr:
2x + (-2x7) = -2
x = +6
oxidation state for C:
2x + [4x(-2)] = -2
x = +3
Product side:
Cr - +3
oxidation state for C:
x + [2x(-2)] = 0
x = +4
America's involvement in World War II had a significant impact on the economy and workforce of the United States. ... American factories were retooled to produce goods to support the war effort and almost overnight the unemployment rate dropped to around 10%.
Answers
Answer:
b
Explanation:
Answer:
b
Explanation:
Estimate the solubility of barium fluoride, BaF2, in a 0.100 M solution of barium nitrate, Ba(NO3)2, given that the K sp of BaF2 is 2.45×10-5.
A)7.83×10-3 M
B)1.57×10-2 M
C)7.83×10-4 M
D)2.45×10-4 M
Answers
Answer:
A) 7.83 x 10^-3 M
Explanation:
7.83 x 10⁻³ M is the solubility of barium fluoride, BaF\(_2\), in a 0.100 M solution of barium nitrate, Ba(NO\(_3\))\(_2\), given that the K sp of BaF\(_2\)is 2.45×10-5. The correct option is option A.
What is solubility?The ability of a material, the solute, to combine with some other substance, the solvent, is known as solubility in chemistry. Insolubility, or the solute's inability to create such a solution, is the opposite attribute. The concentration of a solute inside a saturated solution is typically used to determine how much of a substance is soluble in a certain solvent.
The two compounds are said to be just at solubility equilibrium at this time. There might not be a limit for some solutes or solvents, in which case they two are referred to as "miscible in any quantities." Whereas the solvent is often solid or liquid, the solute may be either a solid, liquid, or gas.
BaF\(_2\)=0.100 M
Ba(NO\(_3\))\(_2\)=0.100 M
K sp of BaF\(_2\)=2.45×10⁻⁵
solubility of barium fluoride=2.45×10⁻⁵×0.100 =7.83 x 10⁻³ M
Therefore, the correct option is option A.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ2
How do atoms react with other atoms to create compounds?
Answers
Answer: They share electrons and become bonded together.
Explanation: Most interactions among atoms take place in the outermost shell of each atom. The number of each electron in this shell determines how an atom combines with other atoms to form compounds. When atoms combine they gain, lose or share electrons in such a way that the outer shells become chemically complete.
is Al+CuCl2 a spontaneous reaction?
Answers
Answer:
No, it isn't a spontaneous reaction.
Explanation:
A spontaneous reaction is a reaction that requires no outside intervention to start.
All chemical reactions require energy.
mark brainliest please!
name the compound
HgCl2
Answers
What quantity measures the amount of space an object occupies?
O Volume
O Temperature
O Mass
O Density
Please hellppp
Answers
" Volume is the amount of space occupied by a substance, while mass is the amount of matter it contains." -What Is the Definition of Volume in Science? - ThoughtCo
The pressure gauge on a compressed air tank reads 43.2 mmHg. What is the pressure in atm? atm
30 points
Answers
The pressure gauge on a compressed air tank that reads 43.2 mmHg is equivalent to 0.057atm.
How to convert pressure units?Pressure in chemistry refers to the force of all the gas particle/wall collisions divided by the area of the wall.
Millimetre of mercury (mmHg) is a unit of pressure equal to the amount of fluid pressure one millimeter deep in mercury at zero degrees Celsius on Earth.
Atmospheric pressure (atm) is the unit of measurement equal to the average air pressure at sea level at a temperature of 15 degrees Celsius. It can be converted as follows:
Divide mmHg value by 760 i.e. 43.2 ÷ 760
43.2mmHg is equivalent to 0.057atm
Learn more about pressure at: https://brainly.com/question/14362967
#SPJ1
Suppose that an aspartic acid (aspartate) residue in the active site of an enzyme was mutated to alanine. As expected, the alanine mutant was inactive, suggesting that the aspartic residue was critical to the catalytic mechanism. Which mutation is most likely to restore wild-type level of activity to the alanine mutant?
a. A to Y
b. A to E
c. A to L
d. A to M
e. A to K
Answers
Answer:
b. A to E
Explanation:
The aspartic acid (aspartate) residue in the active site of the enzyme is likely critical to the catalytic mechanism because it is a charged amino acid and can participate in ionic interactions that stabilize the transition state of the catalyzed reaction. Since the alanine mutant is inactive, we need to introduce a charged amino acid at this position to restore the activity.
Out of the options given, the mutation that is most likely to restore the wild-type level of activity to the alanine mutant is (b) A to E. The glutamic acid (glutamate) amino acid is similar to aspartic acid in its chemical properties, as both are negatively charged amino acids. Therefore, introducing a glutamic acid residue at the mutated position is likely to restore the ionic interaction necessary for catalytic activity.
Please help asap!!!!!!

Answers
Answer:
my increasing the amount of input force inorder to lift the load
What determines the placement of an element on the Periodic table
Answers
Answer:
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number.
Explanation:
Answer: Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number.
Explanation:
What's the coefficient in 4Ca(OH)2 ?
Answers
Answer: Molecular Weight of 4Ca(OH)2 is 296.3707 g/mol
Explanation The molar mass and molecular weight of 4Ca(OH)2 is 296.371.
An organelle that is not found in this illustration of a cell would be
A. the cell wall
B. chromosome
C. mitochondria
D. the cell membrane

Answers
Answer:
The answer is cell wall
Explanation:
Because it is
A student burns 1. 50 mol c3h8 according to the following reaction: c3h8 + 5o2 3co2 + 4h2o. How many grams of carbon dioxide are produced? (molar mass of co2 = 44. 01, molar mass of c3h8=44. 11).
Answers
Answer:it is 5 square c
Explanation:
because c is the best
Consider the two molecules whose structures are shown below.
Which one would you expect to be more water soluble.
Explain your answer.
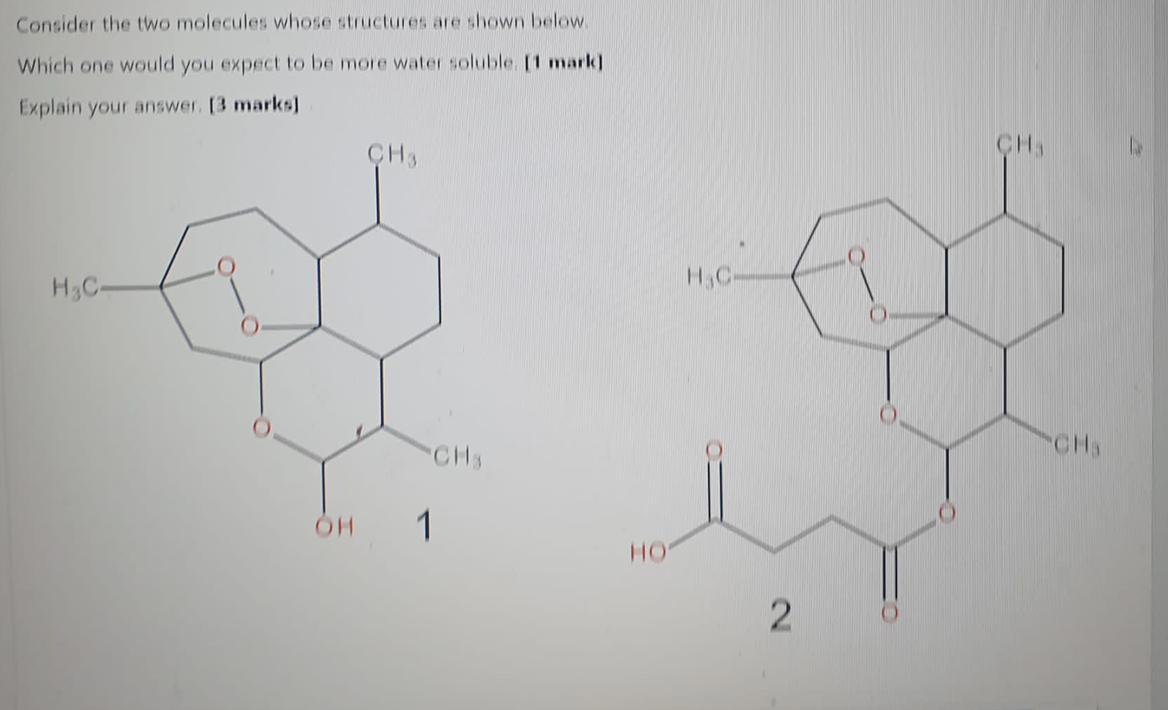
Answers
Molecule 1 will be more water soluble due to the presence of hydroxyl group.
Water solubilityMolecule 1 containing -OH (hydroxyl) will likely be more water-soluble compared to molecule 2 containing -COOH (carboxyl).
The hydroxyl group (-OH) forms hydrogen bonds with water molecules, promoting solubility. These hydrogen bonds allow for strong interactions between the polar hydroxyl group and the polar water molecules.
On the other hand, the carboxyl group (-COOH) is less soluble in water due to its additional carbonyl group (-C=O), which is less capable of forming hydrogen bonds.
Consequently, the presence of the carboxyl group reduces the overall solubility of the molecule compared to the hydroxyl group.
More on water solubility can be found here: https://brainly.com/question/29856613
#SPJ1
3 attempts left
Check my work
Enter your answer in the provided box.
The pressure inside a 1.0 L balloon at 25°C was 750 mm Hg. What is the pressure (in mmHg) inside the
balloon when it is cooled to -65°C and expands to 3.3 L in volume?
mm Hg

Answers
Answer:
shhsss×<×>×××<××××
Explanation:
4×738×8<#329×
Assignment Your Unde a professor in a University has Sent you an touration 6 his Inaugural lectore wate a letter to him, showing appreciation for him on halind gesture and Congratulating! his achievements So far
Answers
In this letter, express gratitude to your uncle, a university professor, for his invitation and congratulate him on his achievements.
Here are the steps to be followed:
Express gratitude and appreciation: Begin the letter by expressing your gratitude for your uncle's kind gesture in inviting you to his inaugural lecture. Show genuine appreciation for his thoughtfulness in including you in such an important event.
Congratulate your uncle on his achievements: Extend your heartfelt congratulations to your uncle for his accomplishments thus far. Acknowledge his hard work, dedication, and commitment to becoming a professor at the university. Highlight specific achievements or milestones that you find particularly impressive.
Share your excitement and anticipation: Express your excitement and anticipation about attending his inaugural lecture. Let your uncle know that you are looking forward to being a part of this significant moment in his career and witnessing his expertise in action.
Offer support and encouragement: Offer words of encouragement and support for your uncle's future endeavors. Let him know that you are proud of him and believe in his continued success. Encourage him to keep pursuing his passion and making a positive impact in his field.
Close the letter with warm regards: End the letter with a closing remark and warm regards. You can use phrases such as "Best regards," "With love," or "Sincerely," followed by your name.
Proofread and revise: Before finalizing the letter, review it for any errors or areas that may need improvement. Ensure that the tone is respectful, appreciative, and heartfelt.
Send the letter: Once you are satisfied with the letter, send it to your uncle either via traditional mail or email, depending on your preferred method of communication.
By following these steps, you can write a thoughtful and appreciative letter to your uncle, expressing your gratitude for his invitation and congratulating him on his achievements.
Know more about Letter here:
https://brainly.com/question/18879087
#SPJ8
I NEED HELP PLS ;-; !!!!
Identify the number of electrons each of the following atoms needs to gain or lose to have a stable outer electron configuration.
a. sodium (Na)
b. strontium (Sr)
c. sulfur (S)
d. astatine (As)
Answers
H2O is a polar liquidOil is a non-polar liquidWhich liquid would be expected to have lower viscosity?wateroil
Answers
Water would be expected to have lower viscosity than oil, because even though water has polar bonds between its molecules, this type of interaction is different with respect to non-polar surfaces.
write down any four uses of solution
.I will mark him\her as brilliant
Answers
Answer:
because it will help u
because it will make u to understand the question very well
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliest