Dr. Oken is testing you again. He wants to see if you know how the neutrons increase in quantity in relation to how the protons increase in quantity. Choose the best explanation to give him.
a The quantity of neutrons is proportional to the megatron. For everyone megatron there is a neutron trying to bring it up from the bottom of the Pacific Ocean
b The neutrons increase almost as quickly as the protons increase in number though there are often less neutrons than protons in the nucleus
c The neutrons increase in number faster than the protons increase
d The number of neutrons increases at the same exact rate as the protons increase in number. You add one proton and you get one neutron. This is true for the entire periodic table.
Answers
For the lighter nuclei up to atomic number 20, the number of neutrons increases at the same exact rate as the protons increase in number. For the heavier nuclei, the increase in the number of neutrons is faster than the protons increase.
What is the neutron-proton ratio?The neutron-proton ratio or N/Z ratio of an atomic nucleus can be described as the ratio of its number of neutrons to its number of protons. Among stable nuclei, the neutron-proton ratio increases with increasing atomic number.
Because electrical repulsive forces between protons with distance are different than strong nuclear force attractions. Proton density in stable larger nuclei should be lower than in smaller stable nuclei.
For each element with atomic number Z up to that of calcium (Z = 20), there exists with N/Z ratio of one, except beryllium (N/Z = 1.25). Uranium-238 has the highest N/Z ratio of 1.587, while lead-208 has the highest N/Z ratio at 1.537.
For the heavier nuclei, the N/Z ratio is greater than 1 so the number of neutrons is greater than the protons.
Learn more about neutron-proton ratio, here:
https://brainly.com/question/17699889
#SPJ1
Related Questions
Calculate the average reading for all three trials for both the wet bulb and dry bulb thermometers. Convert the average
temperatures for both thermometers to Celsius using this formula:
(°F - 32) = °C.
Answers
Answer: °32= 0 °C.
Explanation:
Answer:
Explanation:
Please add the temperature readings from all three trials for both thermometers. Will do the calculation with readings.
which ideas john dalton have
Answers
Answer:
He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, sometimes referred to as Daltonism in his honour.
Explanation:
Answer:
Law of conservation of mass and the law of definite proportions could be explained using the idea of atoms.
Predict whether aqueous solutions of the salts will be acidic, basic or neutral.
CH3NH3I
(C2H5)2NH2I
LiClO4
Ba(F3CCOO)2
KCHCl2CO2
Answers
The salts whose aqueous solutions will be acidic, basic, or neutral are as follows:
CH₃NH₃I: acidic(C₂H₅)₂NH₂I: basicLiClO₄: neutralBa(F₃CCOO)₂: neutralKCHCl₂CO₂: basicThe acidity or basicity of a salt solution depends on the acid-base properties of the ions produced when the salt dissolves in water. In general, if the cation of the salt is the conjugate acid of a weak base and/or the anion of the salt is the conjugate base of a weak acid, then the solution will be acidic. If the cation of the salt is the conjugate base of a strong acid and/or the anion of the salt is the conjugate acid of a strong base, then the solution will be basic. If both the cation and anion are derived from strong acids and bases, then the solution will be neutral.
In the case of CH₃NH₃I, the CH₃NH₃⁺ cation is the conjugate acid of a weak base (methylamine), so it will hydrolyze and produce H+ ions, making the solution acidic. For (C₂H₅)₂NH₂I, the (C₂H₅)₂NH₂⁺ cation is the conjugate base of a weak acid (diethylamine), so it will hydrolyze and produce OH⁻ ions, making the solution basic. LiClO4 and Ba(F₃CCOO)₂ both produce ions derived from strong acids and bases, so their solutions will be neutral. KCHCl₂CO₂ produces the CHCl₂CO₂⁻ anion, which is the conjugate base of a weak acid (dichloroacetic acid), so it will hydrolyze and produce OH- ions, making the solution basic.
To know more about the Aqueous solution, here
https://brainly.com/question/12911927
#SPJ4
Explain the differences in charges and masses, of any, for atoms with different numbers of neutrons
Answers
Answer:
atoms of the same element that have different masses due to their
varying numbers of neutrons
Explanation:
The mass number of an atom is the sum of number of protons and number of neutrons. Whereas, charge is acquired by the lose or gain of electrons.
What is atomic mass?The atomic mass describes how much an atom weighs. The mass of an atom is usually take as relative mass where the mass of an atom is determined relative to the mass of carbon -12.
Mass number describes the sum of number of protons and neutrons. The mass of an atom is mainly contributed by the nucleus thats why take the ,mass number as its mass.
An atom gains positive charge when it loses one or more electron. For example hydrogen with 1 proton and no neutrons will lose its one electron and acquire a positive charge.
When an atom gains an electron it acquire a negative charge. Consider oxygen where 8 neutron and 8 electrons (6 valence electrons) are there with a mass of 16 g/mol. It acquires 2 units of negative charge by gaining two electron. Since it needs two ore electron to attain octet.
Similarly a nitrogen atom with mass of 14 g/mol and 5 valence electrons acquire a -3 charge since it need 3 more electron to attain octet.
To find more on charge, refer here:
https://brainly.com/question/19886264
#SPJ2
which dot and cross diagram is incorrect?

Answers
The dot structure that can be shown to be incorrect is the dot structure that has been shown by option A
What is the dot structure?The Lewis structure is based on the concept that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration similar to that of a noble gas. In the Lewis structure, the valence electrons of the atoms are represented as dots around the symbol of the atom.
We can see that in the dot structure that is in option A the both atoms are coming from the hydrogen atoms and shoud not be differently marked.
Learn more about Lewis structure:https://brainly.com/question/32988499
#SPJ1
PLEASE HELP SOMEONE !!!!!!!!!!!!!!!!!!!

Answers
Answer:
sodium
Explanation:
sodium has 11 electrons.
A scientist provides 454 kJ of energy to a covalent compound in order to start a reaction. As a result, heat is released. Which statement is true about this experiment?
The amount of energy produced is more than 454 kJ.
The amount of energy produced is equal to 454 kJ.
The amount of energy produced is less than 454 kJ.
The amount of energy produced is at least double that of 454 kJ.
Answers
Answer:
The amount of energy produced is less than 454 kJ.
Explanation:
I do not know but if am wrong you can correct me. I need your help for business, english and chemistry questions on my profile. So can you and the other person who commented and go on my profile please...? the due date already gone 12 days ago.
The salts of carboxylic acids, such as sodium benzoate, are often used in foods as A) preservatives. B) colorings. C) sweeteners. D) flavor enhancers
Answers
The salts of carboxylic acids, such as sodium benzoate, are often used in foods as preservatives. The correct answer is A) preservatives.
Salts of carboxylic acids, including sodium benzoate, are commonly used as preservatives in food. They help inhibit the growth of bacteria, fungi, and other microorganisms, thus extending the shelf life of various food products. Preservatives like sodium benzoate are particularly effective in acidic environments, such as soft drinks, fruit juices, and pickled foods.
While some food additives may serve multiple purposes, in the case of salts of carboxylic acids like sodium benzoate, their primary function is as a preservative rather than a coloring, sweetener, or flavor enhancer.
Learn more about preservatives here:
https://brainly.com/question/28123485
#SPJ11
Please help due today
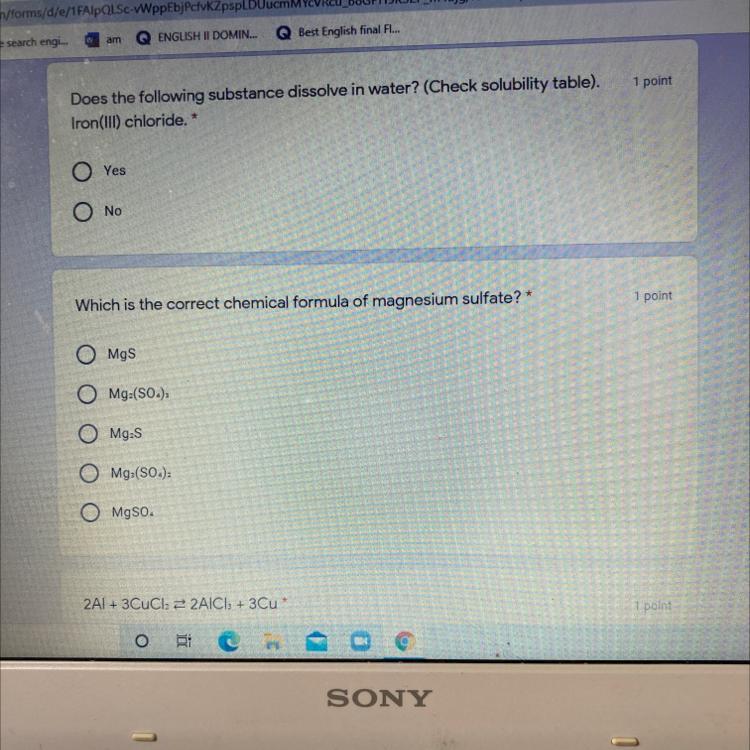
Answers
Answer:
the answer for the magnesium sulphate is MgSO4
Answer:
For the first one it's no.
On your Second question it's MgS.
reaction: bromination which compound (a, b, or c) reacts the fastest? which compound (a, b, or c) reacts the slowest? o nhcch3 f
Answers
Based on the given options, Compound B would react the fastest, Compound C would react the slowest, and Compound A would have intermediate reactivity.
Based on the given reaction, bromination, the reactivity of the compounds can be determined based on the presence of electron-donating or electron-withdrawing groups.
Compound A: C(CH₃)₃
Compound B: CN
Compound C: OH
In bromination reactions, electron-donating groups increase the reactivity, while electron-withdrawing groups decrease the reactivity.
Fastest reaction: Compound B (CN)
The presence of a cyano group (-CN) in Compound B is an electron-withdrawing group, which increases the reactivity towards bromination. Therefore, Compound B would react the fastest.
Slowest reaction: Compound C (OH)
The presence of a hydroxyl group (-OH) in Compound C is an electron-donating group, which decreases the reactivity towards bromination. Therefore, Compound C would react the slowest.
Compound A (C(CH₃)₃) does not have any functional groups that significantly influence the reactivity towards bromination. It may have intermediate reactivity.
So, based on the given options, Compound B would react the fastest, Compound C would react the slowest, and Compound A would have intermediate reactivity.
To know more about electron-withdrawing groups:
https://brainly.com/question/30891937
#SPJ4
--The question is incomplete, the given complete question is:
"(C(CH₃)₃)CN OH A B с Reaction: Bromination Which compound (A, B, or C) reacts the fastest? Which compound (A, B, or C) reacts the slowest? CH₃ H₃C H₃C CH₃ N-C-CH₃ H₃C-N-CH₃ Reaction: Bromination Which compound (A, B, or C) reacts the fastest? Which compound (A, B, or C) reacts the slowest?"--
ILL GIVE BRAINLIST Mrs. Kucalaba downloaded some science music from the Internet onto her iPhone. She listened to the science music while she was building a deck in her mom’s yard.
Which energy transformation explains how Mrs. Kucalaba was able to listen to music on her iPhone while building a deck in her mom’s yard?
Question 19 options:
Light energy was transformed to chemical energy
Chemical energy was transformed to sound energy
Sound energy was transformed to chemical energy
Chemical energy was transformed to heat energy
Answers
Find the grams in 5.26 x 10^-4 mol of HC2H3O2.
Answers
The question requires us to calculate the mass, in grams, contained in 5.26 x 10^-4 mol of HC2H3O2.
To solve this question, first we need to calculate the molar mass of the compound, considering the number of atoms of each element, and then relate the value obtained with the number of moles given (5.26 x 10^-4 mol).
First, to calculate the molar mass of the compound, let's consider the following atomic masses:
atomic mass of C = 12.01 u
atomic mass of H = 1.007 u
atomic mass of O = 15.99 u
Next, we calculate the molar mass. To do that, we need to consider the number of atoms of each element: according to the chemical formula, there are 2 atoms of C, 4 atoms of H and 2 atoms of O:
molar mass (C2H4O2) = (2 * 12.01) + (4 * 1.007) + (2 * 15.99) = 60.03 g/mol
Now, we know that there are 60.03 g for each mol of the compound. With that information, we can estabilish the following relation to calculate the mass contained in 5.26 x 10^-4 mol of the compound:
1 mol --------------------- 60.03 g
5.26 x 10^-4 mol ----- x
Solving for x, we have:
\(x=\frac{(5.26\times10^{-4}\text{ mol)}\times(60.03\text{ g)}}{(1\text{ mol)}}=0.03158\text{ g}\)Therefore, there are 0.03158 g of HC2H3O2 in 5.26 x 10^-4 mol of this compound.
Step 2: Show the conversions required to solve this problem and calculate the grams of KCIO3.
20.8 g 0₂ ×
122.55 g KCIO,
32.00 g 0₂
74.55 g KCI
X
Answer Bank
1 mole KCIO3
1 mole 0₂
1 mole KCI
X
2 moles KCIO,
3 moles 0₂
2 moles KCI
= g KC103

Answers
The grams of KCIO3 are 4.05 g.
What is the purpose of using dimensional analysis in this problem?Dimensional analysis is used to cancel units and convert between different quantities (moles, grams, etc.) in a systematic and logical way. By using conversion factors, we can ensure that our calculations are accurate and that we arrive at the correct units for the final answer.
Why do we need to convert the moles of O2 to moles of KCIO3 before finding the grams of KCIO3?We need to convert the moles of O2 to moles of KCIO3 because we are ultimately interested in finding the mass of KCIO3. By using the conversion factor of 2 moles KCIO3/3 moles O2, we can relate the two quantities and determine the number of moles of KCIO3 required to react with the given mass of O2.
To solve the problem, we need to use the given conversion factors and dimensional analysis to cancel units and find the grams of KCIO3.
Step 1: Write down the given conversion factors:
1 mole KCIO3 = 122.55 g KCIO3
1 mole O2 = 32.00 g O2
2 moles KCIO3 = 3 moles O2
2 moles KCIO3 = 2 moles KCI
Step 2: Write down the given mass of O2 and use it to find the moles of KCIO3:
0.8 g O2 × (1 mole O2/32.00 g O2) × (2 moles KCIO3/3 moles O2) = 0.0333 moles KCIO3
Step 3: Convert the moles of KCIO3 to grams:
0.0333 moles KCIO3 × (122.55 g KCIO3/1 mole KCIO3) = 4.05 g KCIO3
Learn more about moles here:
https://brainly.com/question/26416088
#SPJ1
Write the skeleton equation for each of the following reactions. Then balance each of the following chemical equations. 1. Hydrogen + oxygen water 2. Iron (III) oxide + hydrogen water + iron 3. Sodium + water sodium hydroxide + hydrogen 4. Copper + Oxygen Copper (II) Oxide 5. Potassium iodide + chlorine potassium chloride + iodine 6. Chromium + tin (IV) chloride chromium (III) chloride + tin 7. Magnesium + copper (II) sulphate magnesium sulphate + copper
Answers
Balanced and skeletal equations are as following chemical equations. The number of atoms in each element on the reactant and product sides of a chemical reaction should match in order for the reaction to be considered balanced. The number of atoms on the reactant side and the product side of a skeletal equation are not equal.
Skeletal equation-
H₂ + O₂ → H₂O
Fe₂O₃ + H₂ → Fe + H₂O
Na + H₂O → NaOH + H₂
CuO+ O- →CuO
KI + Cl₂→ KCl+ I₂
Cr+ SnCl₄ → Sn + CrCl₃
Mg + CuSO₄ → MgSO₄ + Cu
ZnSO₄+ SrCl₂→ SrSO₄ZnCl₂
NH₄Cl + Pb(NO₃)₂→ PbCl₂ + NH₄NO₃
Fe(NO₃)₃ + MgS → Fe₂S₃ + Mg(NO₃)₂
Balanced Equation-
2H₂ + O₂ → 2H₂O
Fe₂O₃ + 3H₂ → 2Fe + 3H₂O
2Na + 2H₂O → 2NaOH + H₂
CuO+ O- →CuO
2KI + Cl₂→ 2KCl+ I₂
4Cr+ 3SnCl₄ → 3Sn + 4CrCl₃
Mg + CuSO₄ → MgSO₄ + Cu
ZnSO₄+ SrCl₂→ SrSO₄ZnCl₂
NH₄Cl + Pb(NO₃)₂→ PbCl₂ + NH₄NO₃
Fe(NO₃)₃ + MgS → Fe₂S₃ + Mg(NO₃)₂
Learn more about balanced equations here-
https://brainly.com/question/7181548
#SPJ9
Give an example of an extensive property in an intensive property of an iron nail
Answers
An extensive property is a property that depends on the amount of matter in a sample.
An intensive property is a property of matter that depends only on the type of matter in a sample and not on the amount.
Examples of Extensive properties:
The mass of an object is a measure of the amount of matter that an object contains. A small sample of a certain type of matter will have a small mass, while a larger sample will have a greater mass.Another extensive property is volume. The volume of an object is a measure of the space that is occupied by that object.Examples of Intensive properties:
The electrical conductivity of a pure substance is a property that depends only on the type of substance. Silver, gold, and copper are excellent conductors of electricity, while glass and plastic are poor conductors. A larger or smaller piece of glass will not change this property. Other intensive properties include color, temperature, density, and solubility.Read more about Intensive & Extensive properties:
brainly.com/question/1982551
#SPJ1
Please help!! Balancing Nuclear Equations

Answers
The missing part of the equation is found to be 4/2He. Option A
What are nuclear equations?The term nuclear equations have to do with the type of equation in which one type of nucleus is transformed into another sometimes by the bombardment or loss of a particle.
Now the full equation ought to be written as 7/3Li + 1/1H -----> 4/2He + 4/2He. This is because the total mass on the left is 8 and the total charge on the left is 4.
Learn more about nuclear equations:https://brainly.com/question/19752321
#SPJ1
PLEASE HELP! 100 POINS AND I WILL GIVE BRAINLIEST!!!
A plane is experiencing a LOT of problems because of a storm in the area. Is the plane flying in the troposphere or the stratosphere?
Answers
The plane is mostly flying in the troposphere layer. This is because there is more turbulence.
Hope it helps you...
Hope it helps you...Answered by Benjemin ☺️
Answer:
Explanation:
no ask your teacher
is potato chip crumbs made of atoms?
yes or no, explain why.
Answers
Yes. All matter is made of atoms in some way. Even the smallest crumbs are made of quadrillions of atoms. The number of atoms is simply mind boggling.
1 quadrillion = 1,000,000,000,000,000
There are 15 zeros in that number. It might be better to express it as
1 quadrillion = 1.0 * 10^15
Scientific notation compacts large numbers such as this to make it more readable, and allow for faster writing.
What is the pH of a solution whose [H3O+] is
1. 1*10^-9 M?
Answers
9
Explanation:
Therefore, [H3O+]=[H+]=1.0×10−9M [ H 3 O + ] = [ H + ] = 1.0 × 10 − 9 M . Thus, the pH of the solution is 9.
Determine the approximate volume (in mL) of a 0.120 M solution that contains 10.0 g of copper(II) sulfate, CuSO4.
Answers
The approximate volume of a 0.120 M solution that contains 10.0 g of copper(II) sulfate, CuSO4 is 522.14mL.
HOW TO CALCULATE VOLUME:The volume of a solution can be calculated by dividing the number of moles by its molarity. That is;Volume = no. of moles ÷ molarity
According to this question, the Molarity of the copper sulfate solution is 0.120M solution.The no. of moles of CuSO4 = 10g ÷ 159.61g/mol = 0.063mol
Volume = 0.063 ÷ 0.120
Volume = 0.522L
Volume in milliliters = 522.14mL
Therefore, the approximate volume of a 0.120 M solution that contains 10.0 g of copper(II) sulfate, CuSO4 is 522.14mL.
Learn more about Molarity at: https://brainly.com/question/12127540
10 × 10^23 atoms of silver are mixed with 20 × 10^23 atoms of gold. The mixture is melted and cast in the shape of an ornamental fish. During the casting 5.0 percent of the atoms in the mixture are lost. The ornamental casting contains:
Answers
The ornamental casting contains approximately 9.5 × 10^23 atoms of silver and 19 × 10^23 atoms of gold.
To determine the composition of the ornamental casting after the loss of atoms during the casting process, we need to calculate the remaining number of atoms for each element.Given:
Number of silver atoms = 10 × 10^23 atoms
Number of gold atoms = 20 × 10^23 atoms
Percentage of atoms lost = 5.0%
First, we calculate the number of lost atoms for each element:
Number of lost silver atoms = 5.0% of 10 × 10^23 = (5.0/100) * 10 × 10^23 = 0.5 × 10^23 atoms
Number of lost gold atoms = 5.0% of 20 × 10^23 = (5.0/100) * 20 × 10^23 = 1.0 × 10^23 atoms
Next, we subtract the lost atoms from the original number of atoms to determine the remaining atoms for each element:
Remaining number of silver atoms = 10 × 10^23 - 0.5 × 10^23 = 9.5 × 10^23 atoms
Remaining number of gold atoms = 20 × 10^23 - 1.0 × 10^23 = 19 × 10^23 atoms
Therefore, the ornamental casting contains approximately 9.5 × 10^23 atoms of silver and 19 × 10^23 atoms of gold.
For more such questions on casting
https://brainly.com/question/26036098
#SPJ8
vanadium has a radius of 135 pm and crystallizes in a body-centered cubic structure. what is the edge length of the unit cell?
Answers
The edge-length of unit cell in a body-centered cubic structure is given by the formula a=4r /√3 and is found out to be 312.138 pm.
What is unit cell?Unit cell is defined as the smallest repeating unit of crystal which when repeated leads to the generation of a crystal.They have 6 lattice parameters and seven crystal structures.Accordingly, the number of atoms in unit cells is different.
In case of BCC unit cell, edge length is calculated by the formula,a=4r /√3 , substitution of values gives, a=4×135/1.73=312.138 pm.
Thus, the edge length of body-centered cubic unit cell is 312.138 pm.
Learn more about unit cell,here:
https://brainly.com/question/29537966
#SPJ1
how many milliliters of a 2.00 m pb(no3)2 solution will react with 50.0 ml of a 1.50 m kci solution?
Answers
To determine the volume of a 2.00 M Pb(NO3)2 solution that will react with 50.0 mL of a 1.50 M KCl solution, we need to use stoichiometry and the balanced chemical equation. And it is 18.75 mL of a 2.00 M Pb(NO3)2 solution.
Based on the balanced chemical equation:
Pb(NO3)2 + 2KCl → PbCl2 + 2KNO3,
the stoichiometric ratio between Pb(NO3)2 and KCl is 1:2.
Given that the volume of the KCl solution is 50.0 mL and its concentration is 1.50 M, we can calculate the moles of KCl present. Using the concentration and volume, we find that there are 0.075 moles of KCl. Since the stoichiometric ratio is 1:2, the number of moles of Pb(NO3)2 required is half of that, which is 0.0375 moles. Using the concentration of the Pb(NO3)2 solution (2.00 M), we can calculate the volume by dividing the moles by the concentration. This gives us a volume of 18.75 mL. Therefore, 18.75 mL of a 2.00 M Pb(NO3)2 solution will react with 50.0 mL of a 1.50 M KCl solution based on the stoichiometry of the balanced chemical equation.
To know more about balanced chemical equation click here: brainly.com/question/29130807
#SPJ11
How many feet are in 3.2 miles
Answers
Answer:
16896
Explanation:
5280*3.2=16896
Answer:
The answer is 16896 feet.Explanation:
1 mile = 5280 feetor, 3.2 miles = 3.2 × 5280 feet =16896 feet. Therefore, the answer is 16896 feet. If you like this answer then mark this answer as BRAINLIEST answer.Thank you ☺️☺️
Which gases in the following list exist as separate atoms: hydrogen, helium, nitrogen, oxygen, neon.
Select one:
a. Hydrogen and helium
b. Helium and nitrogen
c. Helium and neon
d. Oxygen and nitrogen
Answers
helium and neon ( answer C)
I have made a thermometer which measures temperature by the
compressing and expanding of gas in a piston. I have measured that at
100° C the volume of the piston is 20 L. What is the temperature outside if
the piston has a volume of 15 L? What would be appropriate clothing for
the weather?
Answers
The temperature would be 7°C
What is the Charles law?Charles's Law, also known as the Law of Volumes, is a gas law that describes the relationship between the volume and temperature of a gas at constant pressure. It states that, for a fixed amount of gas at a constant pressure, the volume of the gas is directly proportional to its absolute temperature.
We know that;
V1/T1 = V2/T2
V1T2 = V2T1
T2 = V2T1/V1
T2 = 15 * 373/20
= 280 K or 7°C
Learn more about temperature:https://brainly.com/question/11464844
#SPJ1
A sample of gas at 42ºC (315 K) has a volume of 5.2 L and exerts a pressure of 608 mm Hg (0.8 atm). How many moles of gas are in the sample?
Answers
Answer:
0.16 mol
Explanation:
you will use the ideal gas law PV=nRT where R is approximately 0.082 atm×L/mol×K
PV=nRT
n=PV/RT
n=0.8×5.2/0.082×315
n=0.16 mol
displacement reaction with example grade 10
Answers
Answer:
Displacement reactionWhen an atom or group of atoms in a molecule is replaced by another atom or group of atoms, such chemical reaction is called displacement reaction. Displacement reaction is of two types. They are:
Single displacement reactionDouble displacement reactionSingle displacement reactionWhen an atom in a molecule is replaced by another atom, such chemical reaction is called single displacement reaction.
For example:
Zinc + Hydrochloric acid → Zinc Chloride + Hydrogen
Zn + 2HCI → ZnCI₂ + H₂
Here, Zn is more reactive than H₂. So, Zn displaces H from the compound HCI.
Double displacement reactionA chemical reaction in which the molecules of the reactants get decomposed and exchange their corresponding ions to give new products is called a double displacement reaction.
For example:
AgNO₃ + NaCI → NaNO₃ + AgCI
Hope this helps..
Best regards!!
What is the best choice of reagents to perform the following reaction? CI COOH ? CH3OH, H2SO4 , NaCN followed by H30+ O Mg, followed by CO2, then H30+ O HCOOH, H20, heat
Answers
Chlorobenzene can be converted into benzoic acid using Grignard reagent. Thus, treating with Mg metal in dry ether followed by carboxylation and then hydrolyising the product gives benzoic acid.
What is Grignard reagent?Grignard reagents are alkyl magnesium halides with the general formula RMgX. They can be prepared by treating alkyl halides with Mg metal in presence of dry ether.
Chlorobenzene when treated with Mg gives MgCl substituted benzene ring which upon carboxylation using carbon dioxide followed by hydrolysis gives benzoic acid.
Therefore, the reagents needed for the conversion of chlorobenzene to benzoic acid are Mg, CO₂ and water.
To find more on Grignard reagents, refer here:
https://brainly.com/question/14702056
#SPJ1

Zinc is most readily found in protein-rich foods such as shellfish, meats, poultry, and dairy products. Vegetarian sources such as legumes and whole grains offer zinc; however, their ______A. OxylateB PhytateC fiberD. Nitrigen
Answers
Answer: phytate i believe is the answer