Draw the Lewis structure for BO3 3−.
a) Indicate how many single bonds are connected to the central atom:
b) Indicate how many double bonds are connected to the central atom:
Answers
The Lewis structure of BO3 3− is as follows: The number of single bonds connected to the central atom is two.The number of double bonds connected to the central atom is one.
The Lewis structure of BO3 3- can be determined by following a set of rules that govern how valence electrons are arranged around atoms in a molecule. In this case, BO3 3- has a central boron atom (B) surrounded by three oxygen atoms (O) with a negative charge (3-).
To draw the Lewis structure, we first count the total number of valence electrons in the molecule. Boron has 3 valence electrons, and each oxygen has 6 valence electrons, giving a total of 3 + (3 x 6) + 3 = 24 valence electrons.
Next, we arrange the atoms in a way that satisfies the octet rule, which states that atoms tend to gain or lose electrons to achieve a full outer shell of 8 electrons. Since boron only has 3 valence electrons, it cannot form an octet on its own, so it must share electrons with the oxygen atoms.
One possible Lewis structure for BO3 3- is as follows:
mathematica
Copy code
O O
\\ //
B
|
O
In this structure, boron forms two single bonds with two of the oxygen atoms, and one double bond with the third oxygen atom. Each oxygen atom also has a lone pair of electrons, giving them a total of 8 electrons in their outer shell.
Therefore, the number of single bonds connected to the central atom (boron) is two, and the number of double bonds connected to the central atom is one.
To learn more about octet rule, refer below:
https://brainly.com/question/865531
#SPJ11
Related Questions
When you lie down on a mattress the force of ______ pulls your mass towards the floor, while the force of the mattress pushes back up resulting in _______ forces, which allow you to sleep comfortably.
Answers
Answer:
1 gravity 2 counteractive
Explanation:
10. What do you have to do to the coefficients of equation I below to get to equation II?
i. 2 SnO2 + 4 H2 2 Sn + 4 H2O
ii. SnO2 + 2 H2 Sn + 2 H2O
a) Both equation I and II are balanced, but equation I is the correct way to write the balanced equation.
b) Can you divide equation II by another factor and still have it be correct? Why or why not?
c) In a complete sentence, write down a method you could use to determine if an equation is written in the correct way.
Answers
1. We have to divide the coefficient of equation I by 2 to get equation II.
a. Both equations are balanced but equation II is the correct way to write a
balance equation.
b. It will be correct if we divide equation II coefficient by another factor
but we will get fractions as coefficient. For example
\(\frac{1}{2}\)SnO₂ + H₂→ \(\frac{1}{2}\)Sn + H₂O
c. The correct method is checking if the number of moles of element on
the reactant side is same as the those on the product side. And the
conventional way is making sure the coefficients are whole numbers.
i. 2SnO₂ + 4H₂ → 2 Sn + 4H₂O
ii. SnO₂ + 2H₂→ Sn + 2H₂O
We have to divide the coefficient of equation I by 2 to get equation II.
Both equations are balanced but equation II is the correct way to write a
balance equation.
If we divide the coefficients of equation II by another number, we will not
get a whole number coefficients. The coefficient will be fractions , it will
be correct but its not conventional.
The best method to know if equation is written the correct way is checking
if the coefficient are in the lowest whole number factors.
we also have to check if each moles of elements found in the reactant is
the same in the product side.
read more: https://brainly.com/question/16609984?referrer=searchResults
Answer:We have to divide the coefficient of equation I by 2 to get equation II.
Explanation:
how many number of atoms does these have?

Answers
Answer:
Explanation:
16. 12
17. 8
18. 9
19. 10
20. 5
21. 15
22.8
23. 24
24. 12
25. 3 i guess( some one comment for 25th pls)
26. 2
Which example represents sexual reproduction?
A). lioness giving birth to cubs
B). amoeba dividing to form two amoebas
C). new plant developing from the leaf of an air plant
D). new plant growing from the stem of a strawberry plant
Answers
Answer:
D
Explanation:
lioness giving birthe is not the process
amoeba is asexual
its either c or d but most likely d
Answer:
the answer is (A) the lioness giving birth
Explanation:
sexual reproduction is when two parents of different orientation come together to have a baby. e.g. a male and female having a baby
Asexual is when you only need one parent so like with plants and stuff.
How is the mass of 1 mole of an element determined? O A. It is equal to the atomic mass times Avogadro's number. O B. It is the same as the element's atomic mass, but in grams. O c. It is equal to the atomic number times Avogadro's number. O D. It is the same as the element's atomic number, but in grams.
Answers
Answer:
Avogadro's number is the number of particles in one mole of anything. In this context, it is the number of atoms in one mole of an element. It's easy to find the mass of a single atom using Avogadro's number. Simply divide the relative atomic mass of the element by Avogadro's number to get the answer in grams.
Answer:
D. It is the same as the element's atomic mass, but in grams.
Explanation:
other answer didn't give the actual option lol
four u tubes each have distilled water in the right arm, a solution in the left arm, and a semipermeable membrane between arms.
Answers
We are aware that more charged particles should be present in the most concentrated solutions (for KCl, K+, and Cl-). The increased solute concentration draws the water in via osmosis.
As a result, the water level is lowest on the right side of the U-tube (C and D). However, the left side tube (A, B) has a more concentrated solution as compared to the right side tubes (C, D).
The result is that the tube B solution is the highest concentrated.
Describe osmosis.
Osmosis is the naturally occurring net movement of solvent molecules through a selectively permeable membrane in a direction that tends to balance the solute concentrations on the two sides, from a region of high water potential (region of lower solute concentration) to a region of higher water potential.
To learn more about concentrated solution, visit:
https://brainly.com/question/10720472
#SPJ4
We are aware that more charged particles should be present in the most concentrated solutions (for KCl, K+, and Cl-). The increased solute concentration draws the water in via osmosis.
As a result, the water level is lowest on the right side of the U-tube (C and D). However, the left side tube (A, B) has a more concentrated solution as compared to the right side tubes (C, D).
The result is that the tube B solution is the highest concentrated.
Describe osmosis.
Osmosis is the naturally occurring net movement of solvent molecules through a selectively permeable membrane in a direction that tends to balance the solute concentrations on the two sides, from a region of high water potential (region of lower solute concentration) to a region of higher water potential.
Please help I will mark brainy

Answers
Answer:
the answer is c I took the test
If the concentration of H+ ions in a solution is 3.16 x 10^-4mol/1. Then what is the concentration of OH ions?
A 3.16 x 10^-4 mol/L
B 3.16 x 10^-11 mol/L
C 3.16 x 10^-13 mol/L
D 3.16 x 10^-14 mol/L
Answers
If the concentration of H⁺ ions in a solution is 3.16 x 10⁻⁴mol/l. Then the concentration of OH⁻ ions is 3.16 × 10⁻¹¹ mol/l. This is using ionic product of water.
What is ionic product of water?Pure water has low electrolyte strength. It produces protons and hydroxyl ions when it ionizes itself to a very little degree. Water that has self-ionized can be visualized as:
H₂O(l) (acid) + H₂O(l) (base) ↔ H₃O⁺(conjugate acid) + OH⁻(conjugate base)
It demonstrates that water is both a proton donor and an acceptor.
Only a small fraction of the millions of water molecules—which are only minimally ionized—are broken down into H⁺ and OH⁻ ions. Because 1 litre of water equals 1000cc = 1000g and the molar mass of H₂O equals 18gmol⁻¹, the concentration of unionized water molecules, or [H₂O], remains nearly constant (being equivalent to 1000/18=55.55 moles per litre), i.e., [H₂O]= constant.
Kw=[H₃O⁺][OH⁻]
Alternatively, Kw=[H⁺][OH⁻]
An ionic product of water (Kw) is the new constant, which is a result of the equilibrium constant and water concentration.
The concentration of OH⁻ ions can be calculated from the concentration of H⁺ ions using the expression for the ion product of water (Kw):
Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴ mol/L
Given the concentration of H⁺ ions and presuming that the solution is in equilibrium, we can solve for the concentration of OH⁻ ions:
[H⁺][OH⁻] = 3.16 x 10⁻⁴ mol/L × [OH⁻]
= (1 x 10⁻¹⁴mol/L)/ (3.16 x 10⁻⁴ mol/L)
[OH⁻] = 3.16 x 10⁻¹¹ mol/L
To know more about electrolyte, visit:
https://brainly.com/question/29771118
#SPJ1
.
please tell me if i did it right. did i put the right electric charge
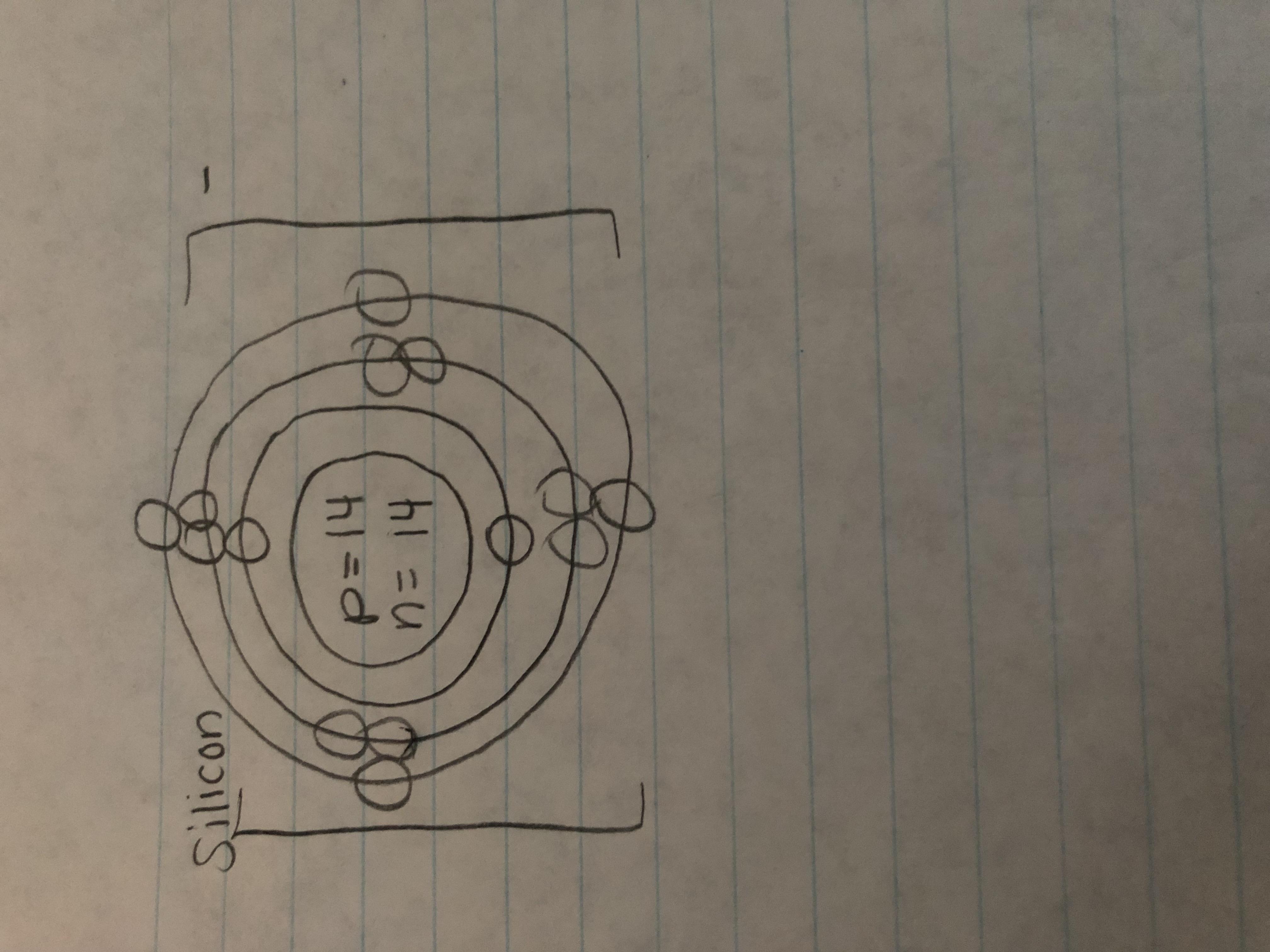

Answers
Answer:
its good but your answer
A 50-gallon drum is being used to concentrate clean water that is flowing into the top of the drum with chlorine.
Water flows in at a rate of 5 gallons per hour, with chlorine concentrated at 3 grams per gallon. Chlorinated water is then being
pumped out at the same rate to keep the drum full of liquid without overflowing. The water is initially clean and contains no
chlorine.
a) Write a differential equation modeling the rate at which chlorine accumulates in the drum, y, in grams, t hours since the concentration process begins.
b) Find any equilibrium point(s) and explain the practical meaning of this value(s).
c) Using the idea of a phase line (do not solve the ODE), describe what we can expect to happen to the amount of chlorine in the tank in the long-run.
Answers
A) The differential equation is dy/dt = (3/50) * 5 - (y/50) * 5.
B) Equilibrium point: y = 30g. It represents the steady-state chlorine concentration where the inflow rate matches the outflow rate.
C) The chlorine amount will approach and stabilize at 30g. No net change occurs as the inflow matches the outflow.
In part A, we are given the information about the rate at which clean water with chlorine is flowing into the drum and being pumped out to maintain the liquid level. The differential equation dy/dt = (3/50) * 5 - (y/50) * 5 models the rate at which chlorine accumulates in the drum over time. The first term represents the inflow rate of chlorine, and the second term represents the outflow rate. By subtracting the outflow rate from the inflow rate, we get the net rate of accumulation of chlorine in the drum.
In part B, we find the equilibrium point(s) by setting the rate of accumulation (dy/dt) to zero and solving for y. The equation (3/50) * 5 - (y/50) * 5 = 0 simplifies to 3 - y/10 = 0, and solving this equation gives y = 30. This means that when the concentration of chlorine in the drum reaches 30 grams, the inflow rate of chlorine matches the outflow rate, resulting in a steady-state concentration.
The practical meaning of this equilibrium value is that the drum will maintain a constant chlorine concentration of 30 grams in the long run, as long as the inflow and outflow rates remain unchanged.
In part C, using the concept of a phase line, we can expect that the amount of chlorine in the tank will approach and stabilize at the equilibrium value of 30 grams in the long run. Since the inflow rate of chlorine is balanced by the outflow rate, there will be no net change in the concentration over time. The system will reach a stable state where the chlorine concentration remains constant. Thus, the chlorine amount will remain at 30 grams indefinitely.
Learn more about differential equation
brainly.com/question/33433874
#SPJ11
What is the final temperature of a 300.0 gram glass of water if it is placed
in the microwave at 22.0°C and it absorbs 5000.0 joules of energy?
Answers
The final temperature of a 300.0-gram glass of water if it is placed
in the microwave at 22.0°C and it absorbs 5000.0 joules of energy is
3.987°C.
What does ' amount of heat gained or lost " means?The equation q = MCT, where m is the mass of the sample, c is the specific heat, and T is the temperature change, can be used to determine the amount of heat that a sample (q) gains or loses. q = mct. liquid mass, m (so this would be in grams) t = temperature change. For example, water's particular constant, C, is 4.2 J g-1 K-1, therefore you just enter that value into the formula in the exam to get the energy of water.
Calculating heat energy involves using the equation Q = MCT. Mass, specific heat, and temperature change are all components of heat energy. Q = MCT. where Q is the heat energy, M is the material's mass, C is the specific heat, and T is the temperature change.
q = m CΔT
5000 J = 300 g × 4.18 J × ΔT
ΔT = 5000/1254
= 3.9872
= 3.987°C.
To know more about the amount of heat gained or lost ,visit:
https://brainly.com/question/13320482
#SPJ9
Electromagnetic waves are used in many technological devices. Describe two examples of how energy from electromagnetic waves is used in technologies to do work. Include at least one wave property that is important for each example.
Answers
Answer:
Electromagnetic waves are ubiquitous in nature (i.e., light) and used in modern technology—AM and FM radio, cordless and cellular phones, garage door openers, wireless networks, radar, microwave ovens, etc. These and many more such devices use electromagnetic waves to transmit data and signals.
Explanation:
i mean there's alot of stuff that have Electromagnetic waves in it i mean alot!
The two examples of how energy from electromagnetic waves is used in technologies to do work are (i) For communication and radar radio waves are used. (ii) For cooking our food microwaves are used.
What is Electromagnetic Wave ?Electromagnetic waves are also called as EM waves are waves that are composed of vibrations between a magnetic field and an electric field. Electromagnetic wave are represented by a sinusoidal graph.
How energy from electromagnetic wave used in technological applications ?For communication and radar radio waves are used. For cooking our food microwaves are used. Infrared waves are used in remote controls. Mobile phones send microwave signals by the atmosphere.
Thus from the above conclusion we can say that The two examples of how energy from electromagnetic waves is used in technologies to do work are (i) For communication and radar radio waves are used. (ii) For cooking our food microwaves are used.
Learn more about the Electromagnetic waves here: https://brainly.com/question/25847009
#SPJ2
Given subsets A and B of Ω, identify all sets in σ(A,B).
Answers
The sets in σ(A,B) are the smallest σ-algebra that contains both A and B.
In probability theory and measure theory, a σ-algebra is a collection of subsets of a given set Ω that satisfies certain properties. The notation σ(A,B) represents the smallest σ-algebra that contains both subsets A and B. This means that σ(A,B) consists of all possible subsets that can be formed by taking the union, intersection, and complement of sets in A and B.
To understand this concept better, let's consider an example. Suppose we have a set Ω = {1, 2, 3, 4} and two subsets A = {1, 2} and B = {2, 3}. The σ-algebra σ(A,B) would include the empty set, the set Ω itself, as well as other subsets such as {1}, {2}, {3}, {1, 2}, {2, 3}, and {1, 2, 3}. It would also include their complements, for example, the complement of {1} would be {2, 3, 4}.
The σ-algebra σ(A,B) is important in probability theory as it allows us to define probability measures and study various properties of events and random variables. By identifying all the sets in σ(A,B), we can determine the range of events that can be analyzed within this framework.
Learn more about σ-algebras
brainly.com/question/32708586
#SPJ11
If I have 3. 9 L of gas at a pressure of 5. 0 atm and a temperature of 50. 0 °C, what will be the temperature of the gas if I decrease the volume of the gas to 2. 4 L and decrease the pressure to 4. 0 atm?
Answers
The temperature of the gas when the volume is decreased to 2.4 L and the pressure is decreased to 4.0 atm is approximately 324.9 K (or 51.75 °C).
To solve this problem, we can use the combined gas law, which relates the pressure, volume, and temperature of a gas:
(P1 × V1) / T1 = (P2 × V2) / T2
where P1, V1, and T1 are the initial pressure, volume, and temperature of the gas, and P2, V2, and T2 are the final pressure, volume, and temperature of the gas.
(5.0 atm × 3.9 L) / (50.0 + 273.15 K) = (4.0 atm × 2.4 L) / T2
Simplifying and solving for T2, we get:
T2 = (4.0 atm × 2.4 L × (50.0 + 273.15 K)) / (5.0 atm × 3.9 L)
T2 ≈ 324.9 K
Therefore, the temperature of the gas when the volume is decreased to 2.4 L and the pressure is decreased to 4.0 atm is approximately 324.9 K (or 51.75 °C).
Learn more about temperature Visit: brainly.com/question/27944554
#SPJ4
what happens to the hexagonal open structure of ice when sufficient pressure is applied to it?
Answers
When sufficient pressure is applied to the hexagonal open structure of ice, the hydrogen bonds between water molecules are compressed and begin to break.
This results in the formation of a denser form of ice known as ice II, which has a different crystal structure than the original hexagonal ice. If even more pressure is applied, ice III, ice IV, and so on can form, each with its own distinct crystal structure. Interestingly, at extremely high pressures, ice can even transform into a non-crystalline form called amorphous ice. This transformation from hexagonal ice to denser forms is an example of a phase transition, which is a common phenomenon in many materials. Understanding the behavior of water and ice under different conditions is important for a wide range of applications, including climate modeling, materials science, and cryogenics.
To learn more about hydrogen bonds click here https://brainly.com/question/17659933
#SPJ11
A 2.50 L sample of butane gas (C4H10), measured at 22.0
oC and 1.20 atm pressure, is combusted completely and
the carbon dioxide gas collected at the same pressure and
temperature. What volume of CO2 is produced?
Answers
Answer:
Same i guess hdjekfhkshfjfkr
The resultant volume of the carbon dioxide gas which is produced by the combustion of butane is 14.4L.
How do we calculate the moles of gas?Moles of any gas will be calculated by using the ideal gas equation as:
PV = nRT, where according to qustion for butane gas
P = pressure = 1.20 atm
V = volume = 2.50L
n = moles = ?
R = universal gas constant = 0.082 L.atm / K.mol
T = temperature = 22 degree celsius = 295.15 K
On putting values we get
n = (1.20)(2.50) / (0.082)(295.15) = 0.124 moles
Given chemical equation is:
C₄H₁₀ + 13/2O₂ → 4CO₂ + 5H₂O
From the stoichiometry of the reaction, it is clear that,
1 mole of C₄H₁₀ = produces 4 moles of CO₂
0.124 mole of C₄H₁₀ = produces 4×0.124=0.496 moles of CO₂
Now we calculate the volume for the carbon dioxide gas at the given pressure and temperature as:
V = (0.496)(0.082)(295.15) / (1.20) = 14.4 L
Hence required volume of CO₂ is 14.4 L.
To know more about ideal gas equation, visit the below link:
https://brainly.com/question/24236411
#SPJ2
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
I’ll mark BRAINLIEST! Please help!

Answers
Answer:
theirs nothing there?!!!??!?!?!??!!?!?!?!?!??!?!??!?!?!??!?!
Explanation:
whys it just a blank picture
The addition of 100 g of a compound to 750 g of CCl4 lowedblue the freezing point of the solvent by 10.5 K. Calculate the molar mass of the compound.
Answers
The molar mass of the compound is 26.25 kg/mol.
The freezing point depression (ΔTf) of a solution is given by the equation:
ΔTf = Kf·m·i
where Kf is the freezing point depression constant of the solvent, m is the molality of the solution, and i is the van't Hoff factor, which is the number of particles that the solute dissociates into when it dissolves in the solvent.
Assuming the solute does not dissociate in \(CCl_4\), i = 1. Therefore, we can rearrange the equation to solve for the molality of the solution:
m = ΔTf/(Kf·i)
We are given that the freezing point depression of \(CCl_4\) (Kf) is 30.0 K·kg/mol. To calculate the molality of the solution, we need to convert the masses to moles. The molar mass (M) of the solute can be calculated as follows:
M = m·(mass of solvent)/(moles of solute)
We can use the formula:
moles of solute = mass of solute / molar mass
To calculate the moles of solute, we need to know the mass of the solute. Since the mass of the solvent is 750 g, the total mass of the solution is:
mass of solution = mass of solvent + mass of solute = 750 g + 100 g = 850 g
Now we can calculate the molality of the solution:
m = ΔTf/(Kf·i) = 10.5 K/(30.0 K·kg/mol·1) = 0.35 mol/kg
Next, we can use the molality and masses to calculate the molar mass of the compound:
M = m·(mass of solvent)/(moles of solute)
M = 0.35 mol/kg·(750 g)/(100 g / M)
M = 26.25 kg/mol
For more question on molar mass click on
https://brainly.com/question/21334167
#SPJ11
A flask that weighs 450 g is filled with 145 ml of benzene. The weight of the flask and benzene is found to be 754 g. From this information, calculate the density of the benzene.
Answers
Answer:
Density, \(d=2.09\ g/cm^3\)
Explanation:
Given that,
Mass of a flask is 450 g
Volume of benzene added to the flask is 145 mL or 145 cm³
The weight of the flask and benzene is found to be 754 g.
We need to find the density of the benzene.
Weight of benzene added = total weight of flask and benzene-mass of flask
m = 754 g - 450 g
m = 304 g
Density = mass/volume
So,
\(d=\dfrac{304\ g}{145\ cm^3}\\\\d=2.09\ g/cm^3\)
So, the density of the benzene is \(2.09\ g/cm^3\).
A single water molecule (H—O—H) is held together by
A) a single covalent bond.
B) a double covalent bond.
C) two polar covalent bonds.
D) hydrogen bonds.
Answers
A single water molecule (H-O-H) is held together by two polar covalent bonds. Option C.
These bonds occur when atoms with different electronegativities share electrons unequally, resulting in a partially positive and partially negative charge on the atoms involved. In the case of water, oxygen is more electronegative than hydrogen, so it pulls the shared electrons closer to itself, giving it a partial negative charge, while the hydrogen atoms have a partial positive charge.
This polarity of the water molecule allows it to form hydrogen bonds with other polar molecules, such as other water molecules, and contributes to the many unique properties of water, such as its ability to dissolve a wide variety of substances and its high surface tension. Therefore, the correct answer is C) two polar covalent bonds.
More on polar covalent bonds: https://brainly.com/question/28295508
#SPJ11
How many molecules of nitrogen gas are found in 0. 045 L of nitrogen gas at STP?
Answers
Answer:
See below
Explanation:
.045 liter / 22.4 l / mole * 6.022 x 10^23 molecules/mole * 2 atoms/molecule =
( * 2 becuase nitrogen gas is diatomic)
When we look at the periodic table of elements, the elements in a have the same number of valence electrons
Answers
Elements in the same group on the periodic table have the same number of valence electrons. The "groups" are the column (or rows). groups are vertically and periods are horizontally.
Rx Ephedrine sulfate (fz. pt = -0.13°C). 2%
Sodium chloride MW 58.5
Purified water qs ad. 30 mL
How much sodium chloride should be used to make this eye
solution isotonic with tears?
the answer is 22
Answers
The correct answer is the amount of sodium chloride needed to make this eye solution isotonic with tears is approximately 1.85 grams. Rounding it up to the nearest whole number gives us the answer as 2. Hence, the correct option is 22.
The given solution is a hypotonic solution as the solution's tonicity is lower than that of the tears. The tears contain 0.9% w/v of NaCl, which is isotonic with tears. So, to make the given solution isotonic, the amount of sodium chloride needs to be added.
The concentration of NaCl in tears is 0.9% w/v. Additional Information: We know that % w/v is the amount of solute present in grams per 100 ml of the solution. Therefore, 0.9% w/v means 0.9 grams of NaCl is present in 100 mL of tears.
To make 30 ml of isotonic solution, we can use the following formula: Equivalent weight of NaCl = 58.5/2 = 29.25 (as NaCl ionizes to give Na+ and Cl- ions)Moles of NaCl required to make 30 ml isotonic solution = 0.9 × 30 / 1000 = 0.027Moles of Na+ and Cl- ions present in 30 mL of isotonic solution = 2 × 0.027 = 0.054
A number of grams of NaCl needed to prepare 30 mL of isotonic solution is calculated as follows:0.054 g = (0.027 x 29.25 x X) / 1000Where X is the amount of NaCl required to make 30 mL isotonic solution. Solving this equation gives us: X = 1.85 g (approx). Therefore, the amount of sodium chloride needed to make this eye solution isotonic with tears is approximately 1.85 grams. Rounding it up to the nearest whole number gives us the answer as 2. Hence, the correct option is 22.
know more about hypotonic solution
https://brainly.com/question/122954
#SPJ11
BEGGING FOR HELP PLEASE
We wish to determine how many grams
of potassium nitrate can form when 100.
mL
of 0.40 M potassium chromate solution
is added to excess silver nitrate.
2AgNO3(aq) + K₂ CrO4 (aq) → Ag2 CrO4(s) + 2KNO3(aq)
In the previous step, you determined
0.040 mol K₂CrO4 react.
The molar mass of KNO3 is 101.11 g/mol.
How many grams of KNO3 can
during the reaction?
Mass (g) KNO3
form

Answers
To calculate the mass of KNO3 created during the reaction, first compute the moles of KNO3 formed. Because the reaction involves a 1:2 mole ratio of K2CrO4 to KNO3, the moles of KNO3 formed can be calculated by multiplying the moles of K2CrO4 by 2.
As a result, 0.040 mol K2CrO4 x 2 = 0.080 mol KNO3. We can determine the mass of KNO3 created now that we know the moles of KNO3 formed by multiplying the moles of KNO3 by its molar mass. Thus, 0.080 mol KNO3 multiplied by 101.11 g/mol equals 8.089 g KNO3.
When 100.mL of 0.40 M potassium chromate solution is introduced to excess silver nitrate, the reaction can produce 8.089 g of KNO3.
Learn more about molar mass at:
https://brainly.com/question/12127540
#SPJ1
draw the molecular orbital diagrams for b2 and calculate the bond order of the molecule. would the molecule be stabilized by adding or removing an electron. explain why.
Answers
To draw the molecular orbital diagram for B2, we need to consider the atomic orbitals of two boron atoms (B) and their interaction.
First, let's consider the electron configuration of boron (B) which is 1s² 2s² 2p¹. Since we have two boron atoms, we can represent their atomic orbitals as follows:
B₁: σ(1s)² σ*(1s)² σ(2s)² σ*(2s)² π(2p)¹
B₂: σ(1s)² σ*(1s)² σ(2s)² σ*(2s)² π(2p)¹
Now, let's fill the molecular orbitals by pairing the electrons:
σ(1s)² σ*(1s)² σ(2s)² σ*(2s)² π(2p)²
The molecular orbital diagram for B2 would look like this:
σ*(2p) π*(2p)
↑ ↑
↑ ↑
σ(2s) ↑ ↑ π(2p)
↑ ↑
↑ ↑
σ(1s) ↑ ↑
Based on the molecular orbital diagram, we can see that there are 2 electrons in bonding orbitals (σ(2s), π(2p)), and no electrons in antibonding orbitals (σ*(2p), π*(2p)). The bond order can be calculated by subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals and dividing the result by 2:
Bond Order = (Number of electrons in bonding orbitals - Number of electrons in antibonding orbitals) / 2
Bond Order = (2 - 0) / 2 = 1
Therefore, the bond order of B2 is 1, indicating a single bond between the two boron atoms.
Now, let's consider whether the molecule would be stabilized by adding or removing an electron. In B2, all the bonding orbitals are fully occupied, resulting in a stable configuration.
Adding an electron would lead to an imbalance and an increase in the number of antibonding electrons, which could destabilize the molecule. Removing an electron would also disrupt the balance between bonding and antibonding orbitals.
Therefore, B2 is already in a stable configuration, and neither adding nor removing an electron would stabilize the molecule further.
To know more about electrons , refer here :
https://brainly.com/question/12001116#
#SPJ11
An iron cube measures 10cm X 10cm X 10cm. What is its volume?
Answers
Answer: 1,000cm
You just multiply them together to get the volume.
The process of evolution results from four known factors, for each listed, describe how those factors were observed in the simulation
Answers
Answer:
objectives
Explanation:
2K(5) + Cl2(g) → 2KCI(S)
what type of chemical reaction is this
Answers
Answer:
Synthesis Chemical Reaction
Explanation:
A+B -> AB
Think of a materials that can produce sound and it can be changed into another form of energy that you would like to create. Draw and illustrate the design of your inverntion. Describe your invention and how it can help people
Answers
One material that can produce sound and be converted into another form of energy is piezoelectric material. Piezoelectric materials have an electrical charge when subjected to mechanical stress.
What is Piezoelectric material?Piezoelectric material produces an electrical charge when subjected to mechanical stress, such as vibrations or pressure. This means that they can convert sound waves into electrical energy, which can then be used to power other devices.
What is the use of piezoelectric material?One potential application of this technology is in developing piezoelectric generators that can be installed in public spaces, such as parks or city streets, to capture the energy from ambient sound waves and convert it into electricity. The electricity generated by these devices could be used to power streetlights, charging stations for mobile devices, or other public amenities.
To learn more about sound, visit here:
https://brainly.com/question/29707602
#SPJ1