Element Arrangement Quick Check
Why is it impossible for an element to have an atomic number of 110.5? (1 point)
A)Atoms with atomic numbers greater than 100 are unstable.
B)Exactly half of the isotopes would need an atomic number of 110, and half would need an atomic number of 111, which is very unlikely.
C)Atoms of an element all have the same whole number of protons.
D)Atoms of an element all have the same whole number of protons and neutrons.
Answers
It is impossible for an element to have an atomic number of 110.5 because atoms of an element all have the same whole number of protons. Thus, option C is correct.
What is an atomic number of an element?The atomic number is the number of protons in the nucleus of an atom. The number of protons defines the identity of an element. The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom.
For example, in a sodium atom, there are 11 electrons and 11 protons.
Thus, the atomic number of Na atom = number of electrons = number of protons is equal to 11.
The atomic number is based only on the number of protons in the nucleus of an atom. Since this is a count of whole numbers, it cannot be a decimal. It's either element 110 or 111, not 110.5.
There is no half proton in the atom, hence we can not have an atomic number of 110.5.
To learn more about atomic number of elements, refer to below link:
https://brainly.com/question/14190064
#SPJ2
Related Questions
The sulfhydryl group of cys-575 in taq polymerase has a pka of 8.47. in a solution of taq polymerase, what fraction of sulfhydryl groups will be deprotonated at ph 7.50?
Answers
At pH 7.50, 0.120 (or 12.0%) of the sulfhydryl groups in Taq polymerase will be deprotonated.
pH is a measure of the acidity or alkalinity of a solution. It is a logarithmic scale that indicates the concentration of hydrogen ions (H+) in a solution. The pH scale ranges from 0 to 14, where a pH of 7 is considered neutral.
Solutions with a pH below 7 are acidic, meaning they have a higher concentration of H+ ions, while solutions with a pH above 7 are alkaline (basic), indicating a lower concentration of H+ ions.
The pKa represents the pH at which half of the sulfhydryl groups will be deprotonated. In this case, the pKa is 8.47.
If the pH is lower than the pKa, a larger fraction of the sulfhydryl groups will be protonated. If the pH is higher than the pKa, a larger fraction will be deprotonated.
Given that the pH is 7.50 (lower than the pKa), we can expect a smaller fraction of the sulfhydryl groups to be deprotonated.
Using the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
In this equation,
[A-] represents the concentration of deprotonated sulfhydryl groups, and
[HA] represents the concentration of protonated sulfhydryl groups.
\(\frac{[A^{-}] }{[HA]} = 10^{(pH - pKa)}\)
\(\frac{[A^{-}] }{[HA]} = 10^{( 7.5 - 8.47)}\)
[A-]/[HA] = 0.120
Learn more about pH, here:
https://brainly.com/question/2288405
#SPJ4
when enzyme is abundant, which is true? group of answer choices a) increasing the concentration of the reactants will increase the reaction rate b) increasing the ph will increase the reaction rate c) increasing the activation energy will increase the reaction rate d) all choices are correct
Answers
When the enzyme is abundant Option A.increasing the concentration of the reactants will increase the reaction rate.
option A is correct
Enzyme awareness increasing enzyme awareness will speed up the reaction, as long as there may be a substrate to be had to bind to. once all the substrate is sure, the reaction will now not speed up, in view that there can be nothing for added enzymes to bind to.
PH is a chief element affecting the interest of enzymes. The ionization of amino acid molecules and atoms takes place while the pH is altered, thereby affecting the shape and form of proteins and therefore disrupting their activities.
Learn more about enzymes here
https://brainly.com/question/1596855
#SPJ4
Name the following three structures.

Answers
The Iupac Name of given three compounds are 3,4,5-trimethylheptane, 2,2,3,3-tetramethylpentane and 2,2- dimethyl-3-ethylpentane.
For (1),
The longest straight chain have 7 carbon compound and having single bond, suffix of this compound is Heptane. Now there are three functional group of methyl there for prefix is trimethyl. Overall the name of compound is 3,4,5-trimethylheptane.
The longest straight chain have 5 carbon compound and having single bond, suffix of this compound is pentane. Now there are four functional group of methyl there for prefix is tetramethyl. Overall the name of compound is 2,2,3,3-tetramethylpentane.
The longest straight chain have 5 carbon compound and having single bond, suffix of this compound is pentane. Now there are three functional group of methyl and ethyl so they write according to alphabetic order. Overall the name of compound is 2,2- dimethyl-3-ethylpentane.
Thus, we concluded that the The Iupac Name of given three compounds are 3,4,5-trimethylheptane, 2,2,3,3-tetramethylpentane and 2,2- dimethyl-3-ethylpentane.
learn more about IUPAC NAME:
https://brainly.com/question/27843604
#SPJ13
What was the effect of the initial temperature on the temperature change of the
water, and why do you think this happened?
Answers
Effect of the initial temperature on the temperature change of the water is because of adding a constant amount of energy the water
Initial temprature is the average temprature of the content of the coldest container to be processed at the time the thermal processing cycle begins and the effect of the initial temperature on the temperature change of the water and this happen because of the cylinder system is adding a constant amount of energy to the water
Know more about water
https://brainly.com/question/28697374
#SPJ1
The molar mass of an unknown compound is 560 g. A sample of the compound consists of 0.900 g of carbon, 0.0751 g of hydrogen, 0.175 g of nitrogen, and 0.600 g of oxygen. What is the the molecular formula of this compound. O C24H₂4N4012 O C24H22N3013 C12H14N₃O5 O C24H₂8 N010
Answers
The molecular formula of the compound is C12H14N₃O5.
To determine the molecular formula of the compound, we need to calculate the empirical formula first, which represents the simplest whole-number ratio of atoms in the compound.
Given the masses of carbon, hydrogen, nitrogen, and oxygen in the sample, we can calculate the moles of each element using their molar masses:
Moles of C = 0.900 g / 12.01 g/mol = 0.0749 mol
Moles of H = 0.0751 g / 1.008 g/mol = 0.0745 mol
Moles of N = 0.175 g / 14.01 g/mol = 0.0125 mol
Moles of O = 0.600 g / 16.00 g/mol = 0.0375 mol
Next, we need to find the simplest ratio of the moles by dividing each value by the smallest value:
Moles of C / 0.0125 = 5.992
Moles of H / 0.0125 = 5.960
Moles of N / 0.0125 = 1.000
Moles of O / 0.0125 = 3.000
Rounding these ratios to the nearest whole number, we get a ratio of 6:6:1:3, which corresponds to the empirical formula C6H6N1O3.
Finally, to determine the molecular formula, we divide the given molar mass of the compound (560 g) by the molar mass of the empirical formula (C6H6N1O3):
560 g / (6 * 12.01 g/mol + 6 * 1.008 g/mol + 1 * 14.01 g/mol + 3 * 16.00 g/mol) ≈ 560 g / 194.19 g/mol ≈ 2.88
Since the result is close to 3, we can multiply the empirical formula by 3 to obtain the molecular formula: C6H6N1O3 * 3 = C18H18N3O9.
However, none of the options provided match the calculated molecular formula.
Learn more about molecular: https://brainly.com/question/1078183
#SPJ11
The molecular formula for the molar mass of the unknown compound is 560g that consists of 0.900 g of carbon, 0.0751 g of hydrogen, 0.175 g of nitrogen, and 0.600 g of oxygen is C₂₄H₂₄N₄O₁₂ (Option A).
To determine the empirical formula, which involves converting the sample into moles. The moles of each element in the compound are calculated using their respective atomic masses.
C = 0.900/12.01 = 0.0749 H = 0.0751/1.01 = 0.0745 N = 0.175/14.01 = 0.0125 O = 0.600/16.00 = 0.0375The smallest number of moles is 0.0125 moles of nitrogen, which is the limiting reagent. As a result, the empirical formula is:
N = 0.0125/0.0125 = 1C = 0.0749/0.0125 = 6H = 0.0745/0.0125 = 6O = 0.0375/0.0125 = 3Therefore, the empirical formula is C₆H₆NO₃.
The empirical formula mass can be calculated by adding the molar masses of each element:
C = 6(12.01) = 72.06 H = 6(1.01) = 6.06 N = 1(14.01) = 14.01 O = 3(16.00) = 48.00Total mass = 140.13
The molecular formula can be determined by comparing the empirical formula mass and the given molar mass. The molecular formula is the same as the empirical formula when the two values are equal. The ratio of the molecular formula mass to the empirical formula mass is equal to the integer value of n (number of empirical formula units):
n = molar mass/empirical formula mass
n = 560/140.13
n = 4
Therefore, the molecular formula is four times the empirical formula: C₂₄H₂₄N₄O₁₂ (Option A).
Learn more about molecular formula: https://brainly.com/question/29435366
#SPJ11
Which of the following statements describes sound energy?
Sound energy does not travel at all.
Sound energy needs a medium to travel through.
Sound energy can travel in empty space.
Sound energy can only travel through air.
Answers
Answer:
C.
Explanation:
a 24.0 g sample contains 14.6 g cl and 9.40 g b. what is the percent composition of chlorine in this sample?
Answers
The percentage of chlorine in this 24.0 g sample, which contains 14.6 g of chlorine and 9.40 g of boron, is 60.83%. Chlorine reacts with organic substances and ammonia, according to its chemical properties (Cl 2 ).
resulting in chloramines or chloro-organics. Chloramines are among the chlorine compounds that exhibit disinfectant capabilities and are detected via a chlorine residue test. When present in wastewater, it serves as a reducing agent.
In this sample, boron makes up 39.17% of the total makeup.
Chlorine weighs 14.60 g in total.
Boron weighs 9.400g in total. Boron is indicated by symbol B.
Sample weight is 24.00 g.
Following are the formulas for calculating chlorine percentage composition:
chlorine content as a percentage = 14.60 / 24 100
proportion of boron is equal to 1460/24.
percentage composition of Boron = 60.833
percentage composition of Boron ≈ 60.83 %
Learn more about chlorine here
https://brainly.com/question/14962130
#SPJ4
Calculate the average atomic mass of rubidium from the data below. Show your work.
Isotope
Rubidium-85
Rubidium-87
Mass
84.9118
86.9092
I
Percent abundance
72.2%
27.8%
Answers
Answer:
avg is 85.4678 atomic mass unit
When excess water is added to a mixture of citric acid and sodium bicarbonate present in a bath bomb, the following reaction takes place
C₆H₈O₇(s) + 3 NaHCO₃(s) + H₂O(l) → Na₃C₆H₅O₇(aq) + 4 H₂O(l) + 3 CO₂(g)
Calculate the volume of CO₂ gas collected over water at 38.0 °C when a mixture of 69.7 g of citric acid and excess sodium bicarbonate is added to a tub of water if the total pressure is 725 torr. The vapor pressure of water at 38.0 °C is 49.7 torr.
Answers
When 55.5 g of citric acid and extra sodium bicarbonate are added to a tub of water at 38.0 °C, 24.9 L of CO2 is accumulated over the water.
How can you determine citric acid's molar mass?Citric acid's molecular weight and formula must be known in order to compute the compound's molar mass. The chemical formula of citric acid is C6H8O6, and it has a molecular weight of 88.15 g/mol. Citric acid's molar mass is 168.15 g/mol as a result.
Finally, we will calculate the amount of CO2 generated. Below is an example to help:
Number of mole (n) = 0.867 moles
Pressure (P) is equal to 725 minus 49.7 torr, or 675.3 / 760, or 0.889 atm.
Temperature (T) = 38 °C = 38 + 273 = 311 K
Gas constant (R) = 0.0821 atm.L/Kmol
Volume of CO₂ (V) =?
PV = nRT
Divide both sides by P
V = nRT / P
V = 24.9 L
V = (0.867 0.0821 311) / 0.889 Hence, 24.9 L of CO2 are collected.
To know more about sodium bicarbonate visit:-
https://brainly.com/question/8560563
#SPJ1
identify ways to reduce the risk of liquid bumping while heating.
Answers
The most common way of preventing bumping is by adding one or two boiling chips to the reaction vessel. However, these alone may not prevent bumping and for this reason it is advisable to boil liquids in a boiling tube, a boiling flask, or an Erlenmeyer flask.
How many grams of copper II chloride (CuCl2) would you need to weigh out to prepare 0.550 L of a 3.00 M Solution? *
2 points
134.45 g
1.65 g
222 g
733 g
Answers
Answer:
1.65 g
Explanation:
is not the right answer
What is the volume of an object that has a mass of 5.80 g and a density of 6.35 g/mL?
Answers
Answer:
1.09 mL
Explanation:
Density is a measure of a substance's mass over its volume.
d = m/v
We can rearrange the equation to solve for volume, using algebra.
v = d/m
Therefore v = 6.35/5.80 = 1.09 mL
Electromagnetic waves used in broadcasting are called?
Answers
Which metalloid has five valence electrons in the fourth electron shell?
antimony
germanium
selenium
arsenic
Answers
Answer:
A- antimony
Explanation:
Answer:
A
Explanation:
Question 4
The analysis of gas and how it behaves has been undertaken to develop several gas laws. Using applicable gas laws establish solutions for the following
a) a mass of gas has a pressure of 450 kPa and temperature of 140°C. The pressure is doubled during a process but the volume remains unchanged. What is the new temperature so cooling systems can be designed?
b) a mass of gas at a temperature of 160°C has a volume of 0.2m³ is cooled down by 110°C with no change in pressure. Calculate the new volume of the gas.
Answers
A mass of gas has a pressure of 450 kPa and temperature of 140°C. The pressure is doubled during a process but the volume remains unchanged.
In order to solve this problem, we need to apply Charles' Law: V1/T1 = V2/T2. Since the volume remains unchanged, we can simplify this to T1/P1 = T2/P2. T1 and P1 are the initial temperature and pressure, respectively. T2 is the unknown final temperature, and P2 is double the initial pressure (i.e., 2P1).
Substituting the given values:140 + 273 = 413 K450 kPa * (2) = 900 kPa413 K/450 kPa = T2/900 kPaT2 = (413 K / 450 kPa) * (900 kPa) = 756 KWe must then subtract 273 to convert from kelvin to Celsius. Therefore, T2 = 483°C, which is the new temperature.
In this case, the gas law to apply is Charles’ law which states that at a constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature. The general equation of Charles' law is V1/T1 = V2/T2, where V is the volume of the gas, T is the temperature, and the subscripts 1 and 2 denote initial and final states, respectively. For our question, since the volume remains unchanged, we can simplify this to T1/P1 = T2/P2. T1 and P1 are the initial temperature and pressure, respectively. T2 is the unknown final temperature, and P2 is double the initial pressure (i.e., 2P1).
Therefore, T2 = (T1 x P2)/P1. We can substitute the given values into the formula and solve for T2 as follows.
140 + 273 = 413 K450 kPa x 2 = 900 kPa
T2 = (413 K x 900 kPa)/450 kPa = 826 K
Subtracting the value of absolute zero (273) from 826, we obtain T2 = 553°C. This is the final temperature of the gas after doubling the pressure.
Learn more about absolute temperature: https://brainly.com/question/16269132
#SPJ11
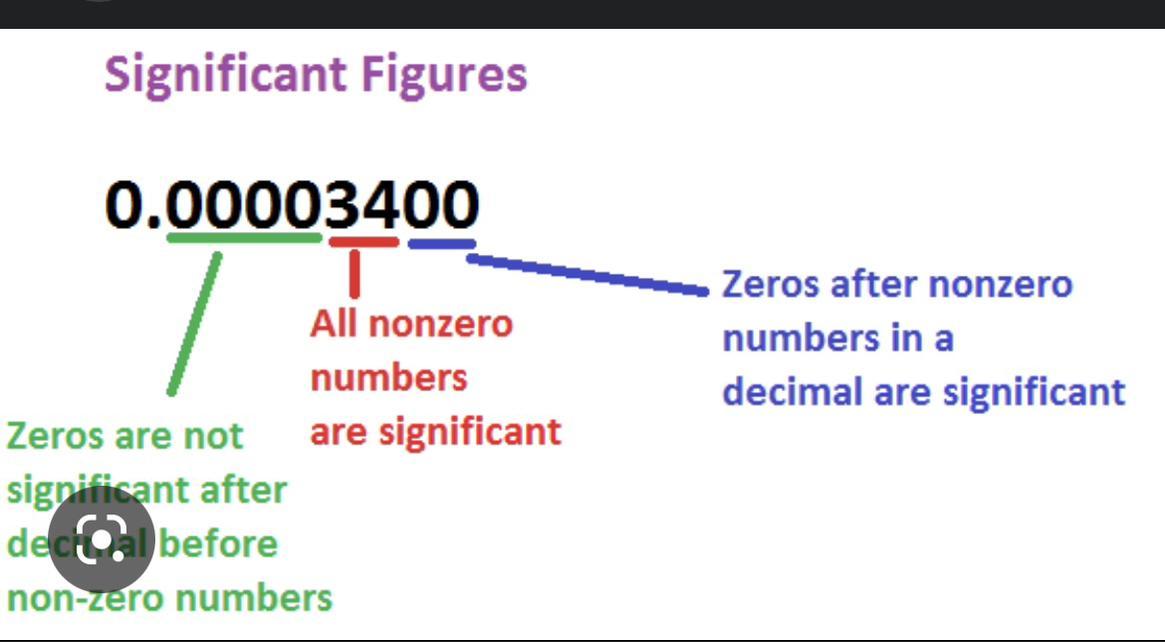
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
5. The Half-life of cobalt-60 is 5 years. What fraction of a sample remains after 20 years?
a.One half
b. One quarter
c.One eighth
d. One sixteenth
Answers
CH4 + 2 02 - CO2 + 2 H2O
How many moles of carbon dioxide are produced from the combustion of 110 g of CH4?
Answers
answer: 6.875 moles
nCH4 = nCO2 = 110/(12 + 4) = 6.875
6.875 moles of carbon dioxide are produced from the combustion of 110 g of CH4.
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
The reaction is
\(\rm CH_4 + 2O_2 = CO_2 + 2 H_2O\)
To calculate the number of moles, we will use the following formula
\(\rm Number\;of \;moles= \dfrac{mass}{molar\;mass}\\\\\\\rm Number\;of \;moles= \dfrac{110g}{12 + 4 } =6.875\)
Thus, the number of moles are 6.875.
Learn more about moles, here:
https://brainly.com/question/15209553
2. How many grams of nitric acid are required to produce 8.75 g dinitrogen monoxide?
4Zn + 10 HNO3 → 4Zn(NO3)2 + N2O + 5 H2O
Answers
Answer:
125 grams of HNO3
Explanation:
(8.75g N2O) x (1/44g N2O) x (10mol HNO3) x (63.02g HNO3/ 1mol HNO3) = 125g HNO3
In this exercise we have to use our knowledge of chemistry to calculate the final amount in grams for nitrogen monoxide, as:
125 grams of HNO3
Thus, to make these calculations it will be necessary to observe the given equation:
\(4Zn + 10 HNO3 \rightarrow 4Zn(NO3)2 + N2O + 5 H2O\)
So after performing the stoichiometry we can perform the calculations as follows:
\((8.75g N2O) * (1/44g N2O) *(10mol HNO3) * (63.02g HNO3/ 1mol HNO3)\\ = 125g HNO3\)
See more about stoichiometry at brainly.com/question/9743981
Starting with one molecule of glucose, glycolysis results in the net production of which of the following sets of energy-containing products?A) 2 NAD+, 2 pyruvate, and 2 ATPB) 2 NADH, 2 pyruvate, and 2 ATPC) 4 NADH, 2 pyruvate, and 4 ATPD) 6 CO2, 2 pyruvate, and 2 ATP
Answers
The net production of energy-containing products from glycolysis, starting with one molecule of glucose, includes 2 NADH, 2 pyruvate, and 2 ATP.
Glycolysis is the metabolic pathway that breaks down glucose into pyruvate. In this process, there is a net production of energy-containing products. Starting with one molecule of glucose, the net energy-containing products produced through glycolysis are 2 NADH, 2 pyruvate, and 2 ATP.
During glycolysis, glucose is converted into two molecules of pyruvate through a series of enzymatic reactions. Along the pathway, two molecules of NAD+ (nicotinamide adenine dinucleotide) are reduced to NADH, resulting in the production of 2 NADH. Additionally, four molecules of ATP are generated, but two ATP molecules are consumed initially, resulting in a net gain of 2 ATP molecules.
To summarize, the net production of energy-containing products from glycolysis, starting with one molecule of glucose, includes 2 NADH, 2 pyruvate, and 2 ATP.
To learn more about heat click here: brainly.com/question/13860901
#SPJ11
The principal, Mrs. Integer tells Mrs. Apex to write down exactly what she is doing step by step. In the scientific method this is called ... a the conclusion. b the data. c the hypothesis d the observation e the procedure
Answers
Answer:
E. the procedure
Explanation:
In scientific experiments, several steps are taken in order to achieve a significant result. However, the steps involved when performing the experiment should be taken note of and written down in a very comprehensible manner. The written step by step process involved in an experiment is called the PROCEDURE.
A procedure should be a guide to performing the experiment by a new experimenter. It should include everything done during the experiment. In this case, Mrs. Integer is telling Mrs. Apex to write down all she is doing during the experiment in a step by step manner. In essence, the principal is simply asking her to write a PROCEDURE.
Which of the following is the correct word equation for the reaction described below? When sulfuric acid reacts with sodium hydroxide, sodium sulfate and water form
Answers
This is an incomplete question, here is a complete question.
Which of the following is the correct word equation for the reaction described below? When sulfuric acid reacts with sodium hydroxide, sodium sulfate and water form.
(1) sulfuric acid + sodium hydroxide → sodium sulfate + water
(2) sulfuric acid → sodium hydroxide + sodium sulfate + water
(3) sodium sulfate + water → sulfuric acid + sodium hydroxide
(4) sodium sulfate + sulfuric acid → sodium hydroxide + water
Answer : The correct option is, (1)
Sulfuric acid + Sodium hydroxide → Sodium sulfate + Water
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
In the balanced chemical equation, the reactants and products are separated by a right arrow.
In the equation, the species present on the left side of the right arrow is known as reactant and the species present on the right side of the right arrow is known as product.
According to the question, when sulfuric acid reacts with sodium hydroxide then it gives sodium sulfate and water as a product.
The complete word equation for the reaction will be:
Sulfuric acid + Sodium hydroxide → Sodium sulfate + Water
Based on the balanced equation : 2 K + Cl2 → 2 KCl If you start with 4 K, how many KCl can you make?
Answers
The law of conservation of mass is obeyed by a balanced chemical equation. The number of moles of KCl produced from 4K is 4KCl.
What is a balanced chemical equation?An equation in which the amount of the reactants and products on both sides of the reaction are equal is defined as the balanced chemical equation. In a balanced equation the number of atoms of each element is same on both sides.
According to the law of conservation of mass, mass can neither be created nor be destroyed. All the balanced chemical equation satisfies this law.
Here when 4K is used the amount of KCl produced is given as:
4K + 2Cl₂ → 4KCl
Here there are 4 'K' atoms and 4 'Cl' atoms. Hence the number of the respective atoms are equal on both sides of the equation.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
Three categories of factors that cause illness or injury are shown here. Match each example on the right to its correct match on the
left.
A baby is born with an abnormal number of chromosomes.
Angela was badly injured in a car accident after her air bag
exploded.
Alex forgot to get a flu shot and missed two weeks of school
with the flu. Answer choices pathogenic agents, genes , environmental factors
Answers
Answer:
Alex forgot to get a flu shot and missed two weeks of school
the lattice energy of sodium chloride, nacl, is -787.5 kj/mol. the lattice energy of potassium chloride, kcl, is -715 kj/mol. in which compound is the bonding between ions stronger? why?
Answers
Because NaCl takes more energy to break it's bigger.
For NaCl, the structure or lattice formation enthalpy is -787 kJ mol-1. Therefore, the lattice dissolution enthalpy is always positive and the lattice formation enthalpy is always negative. The reason that NaCl has the highest lattice enthalpy is that for similar anions, cations with smaller radii have higher lattice enthalpies.
If we want to talk about the amount of energy released when a lattice forms from scattered gas ions we should use the lattice formation enthalpy. MgO has a higher lattice energy because each ion carries two unit charges compared to one in NaCl. NaCl is a compound with higher lattice energy. Description Lattice energy is defined as the energy released when the combination of its constituent ions forms one mole of an ionic compound.
Learn more about The lattice energy here:-https://brainly.com/question/2815368
#SPJ4
What is the formal IUPAC name for CH3CH2CH2CH2C≡CCH3
Answers
The formal IUPAC name for CH3CH2CH2CH2C≡CCH3 is 5-hexyne. This name is derived from the parent hydrocarbon, which in this case is hexane, and indicates that there are five carbon atoms in the chain with a triple bond between the fourth and fifth carbon atoms.
The prefix "hex-" indicates six carbon atoms in the parent chain, and the suffix "-yne" indicates the presence of a triple bond. The "5" indicates the location of the triple bond, which is between the fourth and fifth carbon atoms in the chain. Overall, the name provides a clear and concise way to describe the structure and composition of the molecule according to IUPAC nomenclature conventions.
Visit here to learn more about IUPAC nomenclature : https://brainly.com/question/14379357
#SPJ11
The price of gold (molar mass = 196.97 g/mol) has varied so much over the last 30 years that with $100 you could buy as much as 2.6 troy ounces (81 g) of gold or as little as 0.13 troy ounces (4.0 g). calculate the amount in moles that these two masses of gold represent.
Answers
Answer:
0.41 and 0.02 moles Au
Explanation:
Moles:
81 grams (2.6 troy oz):
mole Au = 81g/(196.97 g/mole) = 0.41 moles Au
4.0g (0.13 oz):
mole Au = 4.0g/(196.97 g/mole) = 0.020 moles Au
After finishing a lab, you have some chemicals left over. You do not want to waste
them, so you carefully pour them back into the container you got them from.
Answers
Answer:
Instead, pour a small amount into a beaker or clean weigh dish. Once you have added a chemical to a container, label it promptly. When you are finished with the experiment, dispose of the excess chemical as chemical waste. Do not simply pour the excess chemical down the sink.
The villi are associated with the ____________ and function in ________________.
Answers
The villi are associated with the small intestine and function in absorption.
What are Villi?Villi are tiny finger-like projections that protrude from the inner lining of the small intestine. Their primary function is to increase the surface area of the small intestine and increase the absorption of nutrients.
What are the functions of the small intestine?The small intestine is the primary site for digestion and absorption. The majority of nutrient absorption takes place in the small intestine. The small intestine is divided into three parts: the duodenum, the jejunum, and the ileum. Food from the stomach is mixed with digestive enzymes and bile in the duodenum.
Most of the nutrients and water are absorbed in the jejunum and the ileum. The small intestine also absorbs vitamins, minerals, and water.The villi are critical for the small intestine's absorptive capacity because they greatly increase the surface area available for absorption. The villi are covered with microvilli, which are even smaller projections that increase the surface area even more.
Learn more about Villi: https://brainly.com/question/1293500
#SPJ11
How many grams are in 1.3 moles of Cr?
Answers
Answer:
40.38 grams
Explanation: