Enantiomers will rotate plane polarized light {{c1::equal amounts in opposite directions}}
Answers
Enantiomers will rotate plane polarized light in equal amounts but in opposite directions.
Enantiomers are a pair of molecules that are mirror images of each other but cannot be superimposed onto each other. Due to their different three-dimensional structures, they have the ability to rotate the plane of polarized light. When a sample of enantiomers is exposed to plane polarized light, each enantiomer will rotate the light in an equal amount but in opposite directions. This phenomenon is known as optical rotation.
Therefore, it can be concluded that enantiomers will rotate plane polarized light in equal amounts but in opposite directions, which is a characteristic feature of these mirror-image molecules.
Enantiomers are pairs of molecules that are mirror images of each other but cannot be superimposed. They have the same chemical properties but can exhibit different optical properties. When plane-polarized light passes through a solution containing one enantiomer, it will rotate the light in a specific direction, either clockwise (+) or counterclockwise (-). When the same light passes through a solution containing the other enantiomer, it will rotate the light in the opposite direction, with the same magnitude.
enantiomers have the unique property of rotating plane-polarized light by equal amounts but in opposite directions, making them optically active compounds.
To know more about three-dimensional, visit
https://brainly.com/question/2400003
#SPJ11
Related Questions
What is the pressure in mm Hg of a 0.025 mole sample of CO2 at 350k in a 2.00L container?
Answers
Answer:
\(273\text{ mmHg}\)Explanation:
Here, we want to get the pressure in mmHg
Using the ideal gas equation:
\(\begin{gathered} PV\text{ = nRT} \\ P\text{ = }\frac{nRT}{V} \end{gathered}\)Where:
P is the pressure in atm which we want to calculate (we would convert to mmHg after calculation)
n is the number of moles which is 0.025 mole
T is the temperature in Kelvin which is 350K
V is the volume which is 2L
R is the molar gas constant which is 0.0821 L.atm/mol.K
Substituting the values, we have it that:
\(\begin{gathered} P\text{ = }\frac{0.025\times0.0821\times350}{2} \\ \\ P\text{ = 0.36 atm} \end{gathered}\)Finally, we have to convert this to mmHg
Mathematically:
\(\begin{gathered} 1\text{ atm = 760 mmHg} \\ 0.36\text{ atm = 0.36 }\times\text{ 760} \\ =\text{ 273 mmHg} \end{gathered}\)Proponents of breast feeding often cite the passing of antibodies to the infant as a benefit. This type of immunity would be considered
artificial active immunity.
natural active immunity.
natural passive immunity.
vaccination.
artificial passive immunity.
Answers
Answer:
Natural passive immunity
Explanation:
Breatsfeeding is natural....
antibodies passes from the parent to the infant...
The infant gets nutrition in which later antibodies will be formed by the nutrients..
How many ml of a 5m solution of sodium borate must be added to a 200 ml solution of 50mm boric acid in order for the ph to be 9.6?
Answers
19 ml of a 5m solution of sodium borate must be added to a 200 ml solution of 50mm boric acid in order for the ph to be 9.6
Here pH=Pka-log[A]/[B]
= 9.6=9.24-log[A]/[B]
= log[A]/[B]=-0.36
Here for ml molarity formula is used
Molarity=mass/volume here mass of sodium borate is 381g/mol and volume of solution is 200ml
mass/volume=381/200=1.905/5.0×50=19ml
Know more about molarity
https://brainly.com/question/28494137
#SPJ4
which liquid property is related to why water beads up on a windshield but acetone does not?
Answers
Surface tension is a liquid characteristic that accounts for why acetone does not form beads on a windshield but water does.
Which liquid property is mentioned here?The amount of force necessary to separate a liquid's surface is measured by its surface tension. High surface tension is caused by the attraction of water molecules to one another rather than to the surface they are in contact with. As a result of this attraction, water molecules limit their contact with the surface area, generating droplets with a high contact angle, which cause water to bead up on hydrophobic surfaces like a windshield. Acetone spreads out on surfaces rather than forming droplets due to its lower surface tension than water, which lowers the contact angle.
Learn more about acetone here:
brainly.com/question/13334667
#SPJ1
what type of reaction is performed with the elephant toothpaste demonstration?
Answers
The reaction performed with the elephant toothpaste demonstration is known as a decomposition reaction.
Decomposition Reaction:The process of breaking down a chemical compound into smaller molecules, atoms, or ions is known as a decomposition reaction. It is also known as analysis or disintegration. A reaction in which a single substance is broken down into two or more simpler substances is known as a decomposition reaction. The elephant toothpaste demonstration is a simple chemical reaction in which hydrogen peroxide breaks down into oxygen gas and water in a matter of seconds.
The formula for hydrogen peroxide is H₂O₂. It is a pale blue liquid that contains hydrogen, oxygen, and water. When you add yeast, soap, and food coloring, the reaction is more exciting. The yeast acts as a catalyst, breaking down hydrogen peroxide into water and oxygen gas. The oxygen gas created causes the soap to foam up, creating the "elephant toothpaste" effect. The chemical reaction that takes place during the elephant toothpaste demonstration can be written as follows:
2H₂O₂(liquid) → 2H₂O (liquid) + O₂ (gas)
This reaction is an example of a decomposition reaction.
To know more about decomposition reaction visit:
https://brainly.com/question/32864042
#SPJ11
How many Coulombs are in 4×10
4
electrons? (6×10
−15C
)
Answers
There are 6.4 × 10⁻¹⁵ Coulombs in 4 × 10⁴ electrons.
To convert the number of electrons to coulombs, we need to first multiply the number of electrons by the charge of a single electron
No. of electrons × Charge of single electron
Charge of single electron = 1.6 × 10⁻¹⁹ coulombs
Calculating using the above formula
we get: 4 × 10^4 electrons × 1.6 × 10⁻¹⁹ C/electron = 6.4 × 10⁻¹⁵ Coulombs
To know more about Electrons refer to this link
https://brainly.in/question/374445
Which choice is a mixture?
carbon
oxygen
air
carbon dioxide
Answers
Answer:
the answer is : air
hope this will help you ❤️
How many moles is 56.0 g of Zn? _______mol
Answers
what are parts of this water wheel system?
Answers
Answer: Explanation:
Water wheels have several important parts that work together (see diagram).
*Flowing water (delivered via a channel called a mill race)
*Large wooden or metal wheels.
*Paddles or buckets (arranged evenly around the wheel)
*Axle.
*Belts or gears.
Please help this is Chemistry!

Answers
Answer:
Number 3
Explanation:
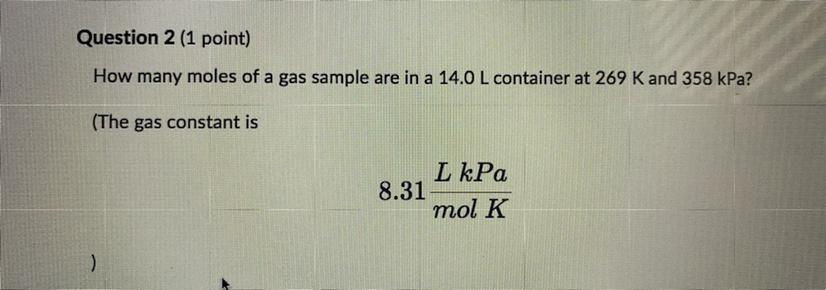
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
A reaction vessel at 1215k contains a mixture of SO2( P = 2. 98 bar) and O2(P = 1. 18 bar). When a catalyst is added, this reaction takes place: 2SO2(g)+O2(g)⇌2SO3(g) At equilibrium the total pressure is 3. 80 bar.
Find the value of k?
Answers
This problem is providing the equilibrium reaction whereby SO3 is produced and asks for the value of the equilibrium constant. After the calculations, the answer turns out to be 0.124.
Chemical equilibrium.In chemistry, when a chemical reaction attempts to go to completion, it might experience a limitation known as equilibrium; a condition where it is not able to proceed any further.
In such a way, for the given reaction:
\(2SO_2(g)+O_2(g)\rightleftharpoons 2SO_3(g)\)
We can write the equilibrium expression as:
\(K=\frac{p_{SO_3}^2}{p_{SO_2}^2p_{O_2}}\)
Hence, given the total pressure at equilibrium, we can add the pressures of all the species at equilibrium including the reaction extent, x:
\(P_{eq}=p_{SO_3}+p_{SO_2}+p_{O_2}\\\\3.80=(2x)+(2.98-2x)+(1.18-x)\)
Thus, we solve for x to obtain:
\(3.80-2.98-1.18=2x-2x-x\\\\-0.36=-x\\\\x=0.36bar\)
Hence, we plug it in into the equilibrium expression to obtain the equilibrium constant as follows:
\(K=\frac{(2x)^2}{(2.98-2x)^2(1.18-x)}\\\\K=\frac{(2*0.36)^2}{(2.98-2*0.36)^2(1.18-0.36)}\\\\K=0.124\)
Learn more about chemical equilibrium: https://brainly.com/question/26453983
consider the following reaction: pbco3(s)←−→pbo(s)+co2(g)
Answers
The given reaction : PbCO₃ (s)⇔ PbO(s) + CO₂ is an example of a decomposition reaction.
A decomposition reaction is a reaction in which two or more different products are formed from a single reactant. It involves the breakdown of a molecule into its simpler forms.
In general, metal carbonates when heated, decomposes to form a metal oxide with the release of carbon dioxide gas. Some metals can readily decompose while some might not.
In the above given reaction, lead carbonate (PbCO₃) which is a white amorphous powdery substance undergoes decomposition to produce the metal oxide i.e lead oxide and releases carbon dioxide gas.
To know more about decomposition reaction here
https://brainly.com/question/11464508
#SPJ4
If you push on a wall with a force of 30 N,the force acting on you from the wall is which of the following
A.40N
B.15N
C.30.N
D.0N
Answers
Answer:
We're working with Newton's Third Law of Motion which states that for every action there is an equal and opposite reaction. When we push on a wall with a force, say 30N, the wall pushes back with the same force.
Explanation:
The force acting on you from the wall will be equal to 30 N. Therefore, option (C) is correct.
What is Newton's third law?According to Newton's 3rd law of motion, when two objects exert force on each other, and these forces are known as interation force pairs.
Force can be defined as a push or pull acting on an object resulting in an interaction between the objects. Force can be defined as an interaction and is classified as: contact force and non-contact force.
According to Newton’s third law of motion when the first object exerts a force on the second object, the first object will experience a force with the same magnitude in the opposite direction.
Given that if we exert the force of magnitude 30 N on the wall. Then the wall will exert the same magnitude of force but in opposite direction. Therefore, the force exerted by the wall on you will be equal to 30 N.
Learn more about Newton's third law of motion, here:
brainly.com/question/23772134
#SPJ2
What assumption is built into the TWA value with regard to years of exposure?
A working career
10 years
20 years
30 years
Answers
The assumption built into the TWA (time-weighted average) value with regard to years of exposure is that the exposure to the content loaded in a particular environment is spread out over a working career, which typically spans around 30 years.
Therefore, the TWA value is based on an average exposure over a period of time, assuming that an individual will work in that environment for a full career length. However, it should be noted that TWA values may vary depending on the specific type of content being measured and the associated health risks.
The assumption built into the Time Weighted Average (TWA) value with regard to years of exposure is "A working career." This typically means that the TWA value is calculated based on a worker's exposure to a substance or hazard over a 40-hour work week for a duration of approximately 30 years, which is considered a standard working career.
Learn more about health risks at: brainly.com/question/12280232
#SPJ11
learn more about the role of secretin in regulating pancreatic bicarbonate ( hco3− ) secretion by completing each sentence using the terms provided.
Answers
Secretin is a hormone released by the duodenal mucosa in response to acidic chyme. It binds to pancreatic ductal cell receptors, initiating a signaling cascade that activates adenylate cyclase.
This leads to increased cyclic AMP (cAMP) levels, promoting the secretion of bicarbonate (HCO3-) ions by pancreatic ductal cells. Bicarbonate ions are transported across the apical membrane into the pancreatic ducts, where they combine with protons to form carbonic acid. Carbonic acid is rapidly converted to water and carbon dioxide by carbonic anhydrase. Carbon dioxide can diffuse into the bloodstream, while remaining bicarbonate ions exit the basolateral membrane into the bloodstream. This secretion of bicarbonate helps neutralize acidic chyme in the duodenum, maintaining a suitable pH for digestive enzyme activity and protecting the intestinal mucosa.
To know more about secretion , visit:
https://brainly.com/question/33292633
#SPJ11
Which of the following samples contains the greatest number of atoms?
a. 9 mole of CO2 b. 10 moles of Xe
c. 11 moles of N2O d. 12 moles of CO
Answers
Answer:
12 moles of CO
Explanation:
According to Avogadro, one mole of a substance, contains the same number of elementary entities as 12g of carbon-12. Now the number of elementary entities (atoms, molecules, ions, particles etc) in any substance is given by the Avogadro's constant.
Now since 1 mole of a substance contains Avogadro's number of atoms, it means that the substance with the highest number of moles will have the highest number of atoms.
With this in mind we can see that 12 moles of CO is expected to contain 72.24 ×10^23 atoms of CO. Hence the answer.
Answer:
c. 11 moles of N2O.
Explanation:
Hello,
In this case, for each substance, we use the Avogadro's number to compute the number of atoms in the given sample:
a.
\(atoms \ C=9molCO_2*\frac{1molC}{1molCO_2} *\frac{6.022x10^{23}atoms\ C}{1molC} =5.4x10^{24}atoms\ C\)
\(atoms \ O=9molCO_2*\frac{2molO}{1molCO_2} *\frac{6.022x10^{23}atoms\ O}{1molO} =1.1x10^{25}atoms\ O\)
\(Total\ atoms=1.1x10^{25}+5.4x10^{24}=1.64x10^{25}atoms\)
b.
\(atoms \ Xe=10molXe *\frac{6.022x10^{23}atoms\ Xe}{1molXe} =6.022x10^{24}atoms\)
c.
\(atoms \ N=11molN_2O*\frac{2molN}{1molN_2O} *\frac{6.022x10^{23}atoms\ N}{1molN} =1.3x10^{25}atoms\ N\)
\(atoms \ O=11molN_2O*\frac{1molO}{1molN_2O} *\frac{6.022x10^{23}atoms\ O}{1molO} =6.6x10^{24}atoms\ O\)
\(Total\ atoms=1.3x10^{25}+6.6x10^{24}=2.0x10^{25}atoms\)
d.
\(atoms \ C=12molCO_2*\frac{1molC}{1molCO_2} *\frac{6.022x10^{23}atoms\ C}{1molC} =7.2x10^{24}atoms\ C\)
\(atoms \ O=12molCO_2*\frac{1molO}{1molCO_2} *\frac{6.022x10^{23}atoms\ O}{1molO} =7.2x10^{24}atoms\ O\)
\(Total\ atoms=7.2x10^{24}+7.2x10^{24}=1.44x10^{25}atoms\)
Thus, we notice 11 moles of N2O have the greatest number of atoms.
Best regards.
7. convert 22.7g to μg
Answers
The 22.7 g in μg ( micro gram ) is 22.7 × \(10^{-6}\) .
We need to convert between units in order to ensure accuracy and prevent measurement misinterpretation.
For example , we do not measure a pencil's length in kilometers . In this situation , one must convert from kilometers ( km ) to centimeters ( cm ) . In most cases, multiplicative conversion factors are used to convert one unit to another of the same quantity .
Sometimes the units of measurement used may not correspond to the standards required for a particular process or application, as well as the measuring choice and convenience. The mass of object in micro gram unit is less than gram .
to learn more about μg please click here ,
https://brainly.com/question/16630356
#SPJ1
Please help! I really need help on this!

Answers
Answer:
I wanna say c
Explanation:
It makes the most sense, because non-metals don't produce an electrical current, and therefore aren't conductors.
Baking soda, NaHCO3, decomposes when it is heated. How much heat will be absorbed by the decomposition of 5.25 moles of baking soda?
Answers
Answer:
The balanced reaction for the decomposition of baking soda is
2 NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
We can find the heat of reaction by using the Hess' Law. This is done by using this formula:
∑(Hf,products) -∑(Hf,reactants) = Heat of reaction
where Hf is the heat of formation. According to literature, these are the heats of formation for each of the compounds in the reaction:
NaHCO3: -947.68 kJ/mol
Na2CO3: -1130.94 kJ/mol
CO2: -393.51 kJ/mol
H2O: -241.8 kJ/mol
Applying Hess' Law:
[1(-1130.94) + 1(-241.82)] + 1(-393.51)] - [2(-947.68)] = 129.09 kJ
Thus, the heat of reaction is 129.09 kJ/mol NaHCO3. Since there is 1.96 mol of NaHCO3, the total heat of reaction is 253.02 kJ
Explanation:
please mark me as brainlest
Collision with energy, Ea, or greater can cause atoms of the colliding molecules to reach the =
Answers
When a collision with energy Ea or greater occurs between molecules, it can cause the atoms of the colliding molecules to reach the activated complex or transition state.
This is the point where the molecules have enough energy to overcome the activation energy barrier and proceed with the chemical reaction. Collision with energy Ea or greater can cause atoms of the colliding molecules to reach the activation energy (Ea) required for a chemical reaction to occur.
When the colliding molecules reach the activation energy, the chemical bonds between the atoms can break and form new bonds, resulting in a chemical reaction. Therefore, collision energy is an important factor in determining the rate of a chemical reaction.
More on energy Ea: https://brainly.com/question/28589626
#SPJ11
sodium and oxygen react to produce Sodium Oxide. How many moles of oxygen are needed to produce 11.5 grams of sodium oxide
Answers
INFORMATION:
We know that:
- sodium and oxygen react to produce Sodium Oxide
-
True or False. Index Fossils are formed from organisms that were alive for short period of geologic time, are extinct, had hard parts, and lived across wide geographic area.
Answers
Answer:
Index fossil, any animal or plant preserved in the rock record of the Earth that is characteristic of a particular span of geologic time or environment.
Explanation:
True
Answer:
Explanation:
A gas at STP has a volume of 37.8 L. If the temperature is raised to 295 K and the pressure is changed to 50.0 kPa, what is the new volume of the gas?
Answers
When a gas at a given temperature and pressure is changed, the new volume of the gas can be calculated using the ideal gas law.
What is the new volume of the gas?The ideal gas law states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.Given the temperature and pressure of the gas, we can rearrange the ideal gas law to solve for V: V = nRT / P. In this equation, n and R are constants. Since the new temperature and pressure are given, we can calculate the new volume of the gas:V = nRT / PV = (n)(0.08206 L•atm/mol•K)(295 K) / (50.0 kPa)V = 45.49 L Therefore, the new volume of the gas at 295 K and 50.0 kPa is 45.49 L, which is an increase of 7.7 L from the original volume of 37.8 L at STP. This is due to the fact that when the temperature and pressure of a gas are increased, the volume of the gas increases as well.To learn more about the ideal gas law refer to:
https://brainly.com/question/25290815
#SPJ1
Which of the following best explain how Earth's rotation relates to living systems?
A. Earth’s rotation severely limits the amount of sunlight hitting a given region, which prevents living systems from surviving
B. By stabilizing temperatures for a given region, earth’s rotation helps sustain living systems in that region
C. Earth’s rotation forces many animals to migrate in order to continuously receive the Sun’s rays
D. Because Earth’s rotation brings darkness to a given region, plants are unable to survive in that region at night
Answers
Answer:
I think it's B
But A seems a good answer too
It might be D
But Imma go for C
Explanation:
how many atoms of boron are present in 8.0*10^-6 moles of boron
Answers
Explanation:
Molar mass of
B
=
10.81
g
⋅
m
o
l
−
1
. If there are 36 odd grams of
B
, there are approx.
7
2
×
N
A
boron atoms, where
N
A
is Avogadro's number.
Explanation:
35.76
⋅
g
10.81
⋅
g
⋅
m
o
l
−
1
×
6.022
×
10
23
m
o
l
−
1
=
?
?
Answer link
MAT
Nov 15, 2015
1.991
×
10
24
Explanation:
Molar mass of B = 10.81g/mol
So, 1 mole of B = 10.81g
and 1 mole =
N
A
=
6.023
×
10
23
By combining,
10.81g of B =
6.023
×
10
23
atoms of B
thus,
35.76g of B =
6.023
×
10
23
10.81
×
35.76
35.76g of B =
1.991
×
10
24
48.176 × 10¹⁷ atoms of boron are present in 8.0 × 10⁻⁶ moles of boron.
Avogadro's NumberAvogadro's number is the number of particles in one mole of substance. 6.022 × 10²³ is known as Avogadro's constant / Avogadro's number.
Avogadro's number = 6.022 × 10²³
How to find the number of atoms ?
Number of atoms = Number of moles × Avogadro's Number
= 8.0 × 10⁻⁶ × 6.022 × 10²³
= 48.176 × 10¹⁷ atoms of boron
Thus, from the above conclusion we can say that 48.176 × 10¹⁷ atoms of boron are present in 8.0 × 10⁻⁶ moles of boron.
Learn more about the Avogadro's Number here: brainly.com/question/859564
#SPJ2
Which of the following conclusions might a scientist make when fossils of two different species of very similar organisms are found in different layers of rock?
Question 2 options:
A. Both organisms had similar behaviors.
B. One organism was prey for the other organism.
C. One organism was ancestor of the other organism.
D. Both organisms lived around the same time.
Answers
Answer:
if its multiple, C and D, if not, C
Explanation:
brainliesttt?
One organism was ancestor of the other organism and both organisms lived around the same time is the conclusion made by scientist.
Who are fossil scientist?Fossil scientist are the specialists in studying the ancient fossils of animals and plants.
If fossils of two different species of very similar organisms are found in different layers of rock then it might be the possibility that one organism was ancestor of the other or both organisms lived around the same time. Because it is not necessary that if two species had the same organism then they have same behavior.
Hence, option (c) & (d) is correct.
To know more about fossil scientist, visit the below link:
https://brainly.com/question/3379389
What is the number of protons, neutrons, and electrons in the following isotopes?

Answers
Answer:
SR: no. of protons=38no. of neutron=51
2. Cr: no. of protons= 24
no. of neutron=28
3. S: number of protons=16
number of neutrons =16
4. BR : number of proton=35
number of neutron =46
Explanation:
In the given questions;
the numerator is atomic mass and in denominator there is atomic number.
Why did it take 2,000 years for scientists to confirm some of Democritus's ideas about?
Answers
Answer:
Explanation:
because it took that long for them to think of one.
What is the molarity of a solution composed of 6.25 g of HCl in 0.300 L ofsolution?
Answers
The concentration is measured by molarity, the formula of molarity is:
\(\text{Molarity (M)=}\frac{mole\text{s of solute}}{liters\text{ of solution}}=\frac{mol}{L}.\)Based on the given data, we've already had the volume in liters and we have to convert 6.25 grams of HCl to moles. We have to use the molar mass of HCl which is 36.4 g/mol (you can find the molar mass using the periodic table). The conversion would be:
\(6.25\text{ g HCl}\cdot\frac{1\text{ mol HCl}}{36.4\text{ g HCl}}=0.172\text{ moles HCl.}\)And the final step is to replace the values we have in the formula of molarity:
\(\text{Molarity}=\frac{0.172\text{ moles}}{0.300\text{ L}}=\text{0}.573\text{ M.}\)The molarity of 6.25 g of HCl in 0.300 L of solution is 0.573 M.