Answers
Related Questions
If a snail crawls 55 inches per day, how many centimeters will he crawl in 23 days?
Group of answer choices
5.0X102 cm
1.06 cm
3.2X103 cm
6.1 cm
2.0X10-3 cm
Answers
If a snail crawls 55 inches per day ,the snail will crawl approximately \(3.2 * 10^3 centimeters.\)
To convert inches to centimeters, we need to use the conversion factor: 1 inch = 2.54 centimeters.
First, let's calculate the distance the snail crawls in inches in 23 days. We can multiply the daily distance by the number of days:
Distance in inches = 55 inches/day × 23 days = 1265 inches.
Now, to convert the distance from inches to centimeters, we multiply the distance in inches by the conversion factor:
Distance in centimeters = 1265 inches × 2.54 cm/inch = 3215.1 cm.
Rounding to the appropriate number of significant figures, the snail will crawl approximately 3215 cm or \(3.2 * 10^3 centimeters.\) in 23 days.
Therefore, the answer is Option 3:\(3.2 * 10^3 centimeters.\)
This means that in 23 days, the snail will crawl approximately\(3.2 * 10^3 centimeters.\)
Know more about centimeters here:
https://brainly.com/question/23883224
#SPJ8
Which one of the following salts is least soluble in water?
1. Na2SO4
2.CaBr2
3. LiCl
4. RbI
5. PbSO4
Answers
A constant electric current deposited 365 mg of Ag in 216 minutes from an aqueous Silver trioxonitrate (v). What is the Current?
Answers
The electric current is 0.025 A
Electric current refers back to the go with the flow of energy in an electronic circuit and to the amount of strength flowing through a circuit. it's far measured in amperes (A). the bigger the cost in amperes, the more energy is flowing within the circuit.
Ag+ + e¯ →Ag
1F deposits 107.87 g/mol (molecular mass) of silver
1F = 96500 C
Let, 107.87 g/mol needed = 96500 C
Number of coulombs required to deposit 0.3650 g of silver =(96500/107.87) 0.3650
Q = 326.5 C
According to Faraday’s law, Q = I x t
I = 326.5 C / (216 x 60 s) = 0.025 A
Learn more about electric current here:-https://brainly.com/question/2984202
#SPJ9
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
A gas occupies 15.2 liters at 0.960 atm. What is the pressure if the volume becomes 35.0 L?.0 mm Hg. What is the volume when the pressure is increased to 90.0 mm Hg?
Answers
When the pressure is increased to 90.0 mm Hg, the volume will be approximately 12.30 liters.
To solve this problem, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Initial conditions:
Volume (V1) = 15.2 liters
Pressure (P1) = 0.960 atm
New conditions:
Volume (V2) = 35.0 liters
Pressure (P2) = 90.0 mm Hg
First, we need to convert the pressure from mm Hg to atm:
P2 = 90.0 mm Hg * (1 atm / 760 mm Hg) = 0.1184 atm
Now we can apply Boyle's Law:
P1 * V1 = P2 * V2
0.960 atm * 15.2 L = 0.1184 atm * V2
V2 = (0.960 atm * 15.2 L) / 0.1184 atm
V2 ≈ 12.30 L
Learn more about Boyle's Law, here:
https://brainly.com/question/21184611
#SPJ1
20.
An aqueous solution that has a hydrogen ion
concentration of 1.0 x 10-8 mole per liter has a pH of
A) 8, which is acidic B) 8, which is basic
C) 6, which is basic D) 6, which is acidic
b
Answers
Answer:
Maybe A
Explanation:
The pH of the aqueous solution is 8 means solution is basic.
How do we calculate pH?pH of any solution will be calculated by using the below equation:
pH = -log[H⁺]
Given that concentration of H⁺ ions = 1.0×10⁻⁸ M
On putting this value in the above equation, we get
pH = -log( 1.0×10⁻⁸ )
pH = -(0-8) = 8
Means the given aqueous soution is basic in nature.
Hence pH is 8 means solution is basic.
To know more about pH, visit the below link:
https://brainly.com/question/26424076
#SPJ2
given two temperatures, 39 °F and 51°F, which if the following correctly compares the number of chances for particals to bounce off each other during chemical reaction?
Same at both
Lower at 39 °F
Lower at 51 °F
Does not depend on temperature
Answers
Answer:
Higher temperature = more bouncing.
So, the correct answer is that there will be less bouncing at 39 degrees F. (Lower at 39 F)
Let me know if this helps!
Need help on chemistry

Answers
Answer:
B
Explanation:
Protons are located in the nucleus, which takes out c
Electrons are in specific shells, so b is the answer
What is meant by H, and 2H?
Answers
Explanation:
H is 1 mole of hydrogen
2H is 2 moles of hydrogen
How does electronegativity affect the polarity of the bond between two
atoms?
A. The more electronegative atom will form the positive pole of a
polar bond.
B. The more electronegative atom will form a nonpolar end of the
bond.
C. The more electronegative atom will make its end of the bond more
negative.
D. Electronegativity differences between the atoms will cancel out
bond polarity
Answers
Answer:
C. The more electronegative atom willl make its end of the
bond more negative
A P E X
The electronegativity affects the polarity of the bond between two atoms, as the more electronegative atom will make its end of the bond more negative. The correct option is C.
What is electronegativity?Electronegativity is a charge that shows the ability of an element to gain electron pairs with other elements during bonding. Electronegativity is altered by the distance between the electron and the nuclei and the atomic number of the element.
Polarity is the state of the atomic body in which it has placed charges in an opposite way to the other atoms so that they can join together.
Thus, the correct option is C. The more electronegative atom will make its end of the bond more negative.
To learn more about electronegativity, refer to the link:
https://brainly.com/question/17762711
#SPJ5
How does the offspring of two parents that reproduce sexually differ from the offspring of a parent who reproduces asexually
Answers
Answer: sexual reproduction results to the offspring having a mix of genetic characteristics of the parents. Where as Asexual offspring tend to look like their parent.
Explanation:
Intercourse leads to the mixing of genetic material. Asexual reproduction is often the parent making a separate copy of themselves.
Which of these is the smallest?
cells
atoms
molecules
cell membranes
Science 7th grade
Answers
Answer: atoms
Explanation: cell membrane is in a cell
How does the energy in chemical bonds affect the energy in a chemical reaction?
Answers
All combustion reactions release energy.
-True
-False
Answers
Answer:
true
Explanation:
All combustion reactions are chemical changes which release energy , hence the statement is true.
What are chemical changes?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical changes,here:
https://brainly.com/question/23693316
#SPJ2
a) At 40 °C, what the saturated solubility level of KCl (aq)? (1 mark)
b) If 40 g of KCl is added to 100 g of water at 90 °C, would the solution be saturated? Explain. (2 marks)
c) Describe two ways in which saturation can be achieved if only 40 g of KCl is added to 100 g of water at 90 ° (3 marks)

Answers
a) The saturated solubility level of KCl (aq) at 40°C is 35.7 g/100 mL of water.
b) No, the solution would not be saturated at 90°C. At this temperature, the solubility of KCl in water is higher than at 40°C. Therefore, more KCl can dissolve in 100 g of water at 90°C than at 40°C. To determine if the solution is saturated, we need to compare the amount of KCl that actually dissolved in the water to the maximum amount of KCl that can dissolve in the water at that temperature. If the amount of KCl that dissolved is less than the maximum amount, then the solution is unsaturated. If the amount of KCl that dissolved is equal to the maximum amount, then the solution is saturated. If the amount of KCl that dissolved is greater than the maximum amount, then the solution is supersaturated.
c) Two ways in which saturation can be achieved if only 40 g of KCl is added to 100 g of water at 90°C are:
i) Heating the solution to a higher temperature: As the temperature of the solution is increased, the solubility of KCl in water also increases. Therefore, by heating the solution to a higher temperature than 90°C, more KCl can be dissolved in the water until the solution becomes saturated.
ii) Allowing the solution to cool slowly: If the solutionis heated to a temperature higher than 90°C and then allowed to cool slowly, the solubility of KCl in water decreases as the temperature decreases. This means that as the solution cools, KCl will begin to precipitate out of the solution until the solution becomes saturated. Alternatively, if the solution is left undisturbed at 90°C and allowed to cool slowly, KCl will begin to precipitate out of the solution as it reaches its saturation point.
A 12.0 m hcl solution has a density of 1.18 g ml. What is the concentration of tthis solution in weight percent and moliarty?
Answers
Answer:
Percentage of HCL = 37.11 % (Approx)
Molarity = 16.17 m (Approx)
Explanation:
Given:
Molarity = 12.0 M of hcl
Density = 1.18 g/ml
Computation:
1L of hcl solution contains = 12 moles
Mass = Moles x Molar mass
Mass of HCl = 12 x 36.5
Mass of HCl = 438 g = 0.438 kg
Mass of 1 L solution = 1.18 x 1,000 ml
Mass of 1 L solution = 1180 g
Percentage of HCL = [438/1180]100
Percentage of HCL = 37.11 % (Approx)
Molarity = 12 / [1.18 - 0.438]
Molarity = 12 / [0.742]
Molarity = 16.17 m (Approx)
Calcium carbonate reacts with HCl according to the following equation:
2HCl(aq)+CaCO3(s)→CaCl2(aq)+H2O(l)+CO2(g)
How many moles of HCl are in 82 mL of 0.14 M HCl?
Express your answer using two significant figures.
Answers
0.0115 mol HCl is the number of moles of hydrochloric acid in 82 mL of 0.14 M HCl. We can round this to two significant figures, which are 0.012 mol.1
The equation for the reaction between calcium carbonate and hydrochloric acid is:2HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)We know that the concentration of the hydrochloric acid is 0.14 M, which means that for every liter of solution, there are 0.14 moles of HCl. Since we have 82 mL of the solution, we need to convert this to liters by dividing by 1000:82 mL ÷ 1000 mL/L = 0.082 L
Now we can use the formula: moles of HCI = concentration × volume in liters moles of HCl = 0.14 M × 0.082 L moles of HCI = 0.0115 mol HCI is the number of moles of hydrochloric acid in 82 mL of 0.14 M HCl. We can round this to two significant figures, which is 0.012 mol.
Know more about calcium carbonate here:
https://brainly.com/question/29906739
#SPJ11
Find volume of an object with the mass of 7.9g in the density of 2.28g/ ml
Answers
Answer:
The answer is
3.46 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \)
From the question
mass = 7.9 g
density = 2.28 g/mL
The volume is
\(volume = \frac{7.9}{2.28} \\ = 3.46491228\)
We have the final answer as
3.46 mLHope this helps you
Suppose a species of snake is living in a certain area. Some of the snakes in the population are black and some are orange. Over time, the area changes and the orange snakes have a harder time blending into the environment. This makes it difficult for the orange snakes to catch food.
According to Darwin’s theory of evolution, what will most likely happen to this snake population over time?
Most orange snakes will survive and reproduce, passing on their traits to their offspring. Few black snakes will remain in the population.
Most black snakes will survive and reproduce, passing on their traits to their offspring. The number of orange snakes in the population will not change.
The black snakes will survive and reproduce, passing on their traits to their offspring. Few orange snakes will remain in the population.
Most orange snakes will survive and reproduce, passing on their traits to their offspring. The number of black snakes in the population will not change.
Answers
Answer:
The black snakes will survive and reproduce while the orange snakes die out.
Explanation:
The orange snakes will be easier to see by their prey and predators, and therefore will die out while the black snakes thrive because of their camouflage.
Answer:
the black snakes will survive and reproduce, therefore, passing their traits to their offspring, while few orange snakes will remain in the population.
Explanation:
what is elimination reaction
Answers
Convert to particles
100 dm3 of Kr**
Answers
100 dm3 of Kr is equal to 100,000,000 cm3 of Kr. Since 1 mole of any gas occupies 22.4dm3 at STP.
What is Kr?Kr is the chemical symbol for krypton, a noble gas element found on the periodic table. It has an atomic number of 36 and an atomic mass of 83.80. Krypton is a colorless, odorless, and tasteless gas that is found in trace amounts in the atmosphere. It is used in a variety of applications, including fluorescent lighting, medical imaging, and welding. Krypton is also used in space exploration, where its inert properties make it a useful gas for deep space probes.
then we can calculate the number of moles of Kr present in the given volume:
Number of moles of Kr = 100,000,000 cm3 / 22.4dm3/mol
= 4,467,849.13 moles of Kr
Since 1 mole of Kr contains 6.022 x 10^23 particles, the number of particles of Kr present in the given volume is:
Number of particles of Kr = 4,467,849.13 moles x 6.022 x 1023 particles/mol
= 2.67 x 1026 particles of Kr
To learn more about Kr
https://brainly.com/question/29078505
#SPJ1
How can you tell that Mercury is not a gas giant?
A.
because it is dense and rocky
B.
because of where it orbits the sun
C.
because it has only one moon
D.
because it has no ring system
Answers
Answer:
The answer to this question is A.
hope it helps :)
please help me with my chemistry homework thank you so muchOptions:miscibleimmisciblepartially miscible
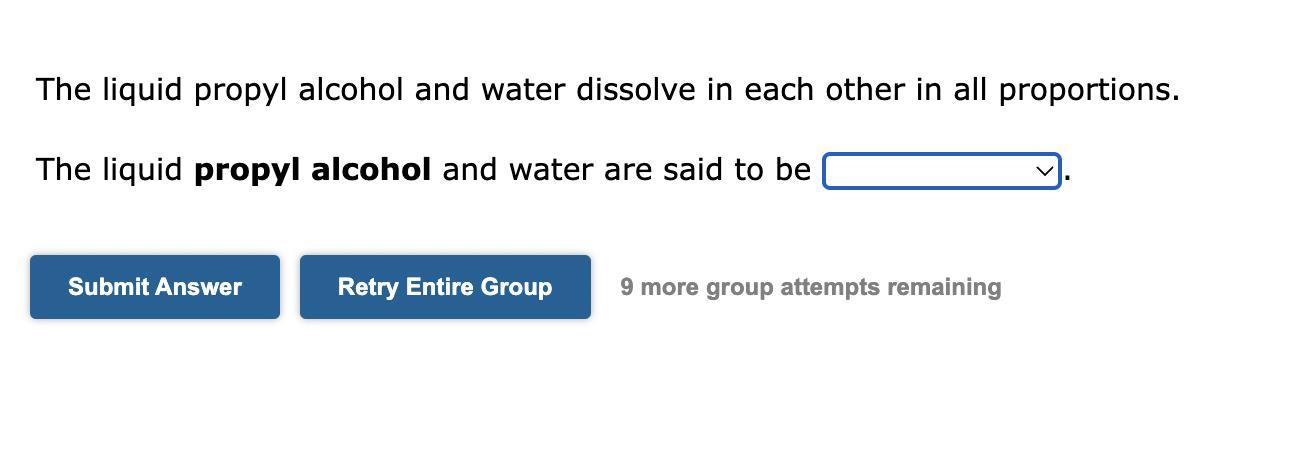
Answers
Chemistry => Solutions => Solubility
A mixture corresponds to joining two or more substances, depending on the behavior of the mixture, it can be said that the substances are miscible, immiscible, or partially miscible.
Immiscible refers to the fact that regardless of the proportion of the substances we will have two phases since the substances do not mix.
Partially miscible means that the substances mix depending on the proportion between them.
Miscible refers to the fact that regardless of the proportion, the substances will always be mixed, therefore we will see a single phase.
Therefore, the correct answer will be: Miscible
Infer: Naos is a star in the Argos constellation. It has a very deep blue color. What do you think is the approximate temperature of Naos?
Answers
Answer:
Being an extreme blue supergiant with 59 solar masses and a surface temperature of over 42000 kelvins, Naos is actually one of the brightest stars in the Milky Way. It's almost 800 000 times as bright as the Sun, although most of that light is ultraviolet.
Explanation:
Wien's displacement law to find the approximate temperature of a star, in this case the Naos temperature would be 64000K, the real temperature is 42000K
Wien's displacement law states that the product of the maximum wavelength emitted by a black body and its temperature is constant
λ_{max} T = 2,898 10⁻³ [ m K]
A black body is a body that absorbs all the radiation that reaches it, which is why it looks black, the cavity inside the black body must be at a fixed mask and it was there so that a thermal equilibrium is established and the radiation can be emitted by the black hole.
The stars approach a black body in the sense that their mission has a similar maximum to that of the black holes, but the temperature is an effective temperature that does not take into account the different processes within the star.
With this wavelength of the emission maximum, the temperature of the stars is calculated, in this case the star Naos is a whitish-blue star, the surface temperature found in tables is 42000K
if we use Wien's displacement law the emission should be
λ_{max} = 2,898 10-3 / T
λ_{max} = 2,898 10-3 / 42000
λ_{max} = 690 nm
this emission corresponds to the color orange
For a blue emission ranging from 450 nm to 500 nm, the temperature of a black body would be
T = 64000K to 57000 K
therefore using wien's displament law the temperature of the star Naos should be approximately 64000k, the fact that the real temperature is lower is because there are several processes with different temperatures inside the star.
In conclusion, Wien's Displacement Law can be used to find the approximate temperature of a star, in this case the Naos Temperature would be 64000K
Learn more about the temperature of the stars here:
https://brainly.com/question/9007054
What volume of hydrogen iodide is produced when 118 liters of hydrogen gas react according to the following reaction? (All gases are at the same temperature and pressure.) hydrogen(g) + iodine(s) hydrogen iodide(g) liters hydrogen iodide
Answers
Answer:
\(V_{HI}=236LHI\)
Explanation:
Hello,
In this case, for the given balanced chemical reaction:
\(H_2(g)+I_2(g)\rightarrow 2HI(g)\)
Thus, since hydrogen and hydrogen iodide are in a 1:2 mole ratio, we can easily compute the yielded volume as shown below:
\(V_{HI}=118LH_2*\frac{2molHI}{1molH_2} \\\\V_{HI}=236LHI\)
Thus, is possible, due to the Avogadro's law which allows to relate moles and volume by a directly proportional relationship.
Regards.
Nobody answering my questions what the answer givng brainliest

Answers
Answer: I think it’s B. Because as you can see from the picture there are layers
Explanation:
I think but just in case ask for a second opinion
Answer:
C. Air bubbles
Explanation:
Cant be A because.... you cant freeze a tempreature
Cant be B because... organism types cant show precipitation patterns.
Cant be D because....well it could be D, but there isnt a way to prove humans increased the tempreature
ITS C hope it helps :)
I also got this question before
Which of the following is/are example(s) of a physical change? *
sugar is dissolved in water
water freezes
tea is brewed
dry ice sublimes
All of these are examples of physical change.
Answers
Anyone want have a baby?
This would be an experiment (;
If u know what I mean
Answers
Answer:
An experiment that could go extremely wrong or just well.
Answer:
Explanation:
Bro huh??? you can't be asking that on brainly bro ;-;
Why doesn’t energy always affect the appearance of the lake?
Please help me I have no clue what to do!?
Answers
Energy will always affect the appearance of the lake because it is made up of water which is subject to physical changes.
Phase transition of matter:Phase transition occurs in the three states of matter which include: solid, liquid and gas.
The lake is made up of water and so therefore would undergo phase transition based on the level of energy.l
During high energy, there is increase in kinetic energy between the molecule of water that makes up for he lake.
This may lead to total evaporation or decrease in the amount of lake water.
During low energy, there is reduction of kinetic energy between the molecule of water that makes up for he lake leading to its freezing.
Therefore, energy will always affect the appearance of the lake because it is made up of water which is subject to physical changes.
Learn more about kinetic energy here:
https://brainly.com/question/8101588
When ur a weak acid is dissolved….

Answers
\(\qquad\qquad\huge\underline{{\sf Answer}}\)
The Correct choice is A. H+ , F- and HF
Since HF is a weak acid, it behaves like an weak electrolyte in water. so, there is incomplete dissociation of HF in water. Therefore, there will be HF molecules present in the solution along with the ions ~