EXTRA CREDIT
How many atoms of H are in 3.00 moles of glucose, C.H2O.? (Hint - this is a 2 step prob)
Show your work
Answers
Answer:
\(\huge\boxed{\sf 36\ H\ atoms}\)
Explanation:
Molecular formula from Glucose:
C₆H₁₂O₆
3 moles of Glucose:
3C₆H₁₂O₆
In 1 mole of Glucose, there are 12 hydrogen atoms.
In 3 moles:
= 12 × 3
= 36 H atoms
\(\rule[225]{225}{2}\)
Related Questions
How many atoms of oxygen are in 5Li2SO4
Answers
Answer:
20 oxygen atoms
Explanation:
Li2SO4 has 4 oxygen atoms, so if there are 5 of that compound then there will be 20 oxygen atoms.
List at least four characteristics of acids
Answers
Answer:
pH>7
sour taste
donate H+ ions
turn litmus paper from blue to red
Explanation:
Answer:
Explanation:
pH < 7.
Sour taste (though you should never use this characteristic to identify an acid in the lab)
Reacts with a metal to form hydrogen gas.
Increases the H+ concentration in water.
Donates H+ ions.
Turns blue litmus indicator red.
the slope of the tangent line at a particular point on a graphical plot of time vs. concentration during a reaction gives the:
Answers
The slope of the tangent line at a specific point on a graphical plot of time vs. concentration during a reaction give the provides the instantaneous reaction rate at that point.
The rate of reaction is an essential property of a chemical reaction, and the knowledge of the reaction rate is necessary to understand the behavior of a reaction. The instantaneous reaction rate at a specific point on a graphical plot of time vs. concentration during a reaction is given by the slope of the tangent line. The slope of the tangent line represents the rate of change of the concentration of the reactants and products at that point. The steeper the slope, the greater the rate of reaction, and the flatter the slope, the lower the rate of reaction.
If the slope is negative, it indicates that the reactants are being consumed at a faster rate than the products are being formed. A graph of concentration vs. time is also known as a kinetic plot. A kinetic plot is an essential tool for studying the kinetics of chemical reactions, it enables us to analyze the rate of reaction at different points in time and provides a visual representation of how the concentration of the reactants and products changes over time. So therefore the slope of the tangent line at a specific point on a graphical plot of time vs. concentration during a reaction give the provides the instantaneous reaction rate at that point.
Learn more about kinetic plot at:
https://brainly.com/question/31326253
#SPJ11
1.) Give the formula for the alkane containing 500 carbons.
2.) Give the formula for the aliens containing 15 carbons.
Answers
\(\mathfrak{\huge{\orange{\underline{\underline{AnSwEr:-}}}}}\)
Actually Welcome to the concept of General organic chemistry.
1.) The formula for alkanes is Cn H2n+2
===> here n = 500, hence we get as,
C500, 2n+2 = 2(500)+2 ==> 1000+2 ==> 1002
hence the formula is ==> C500 H1002
2.) The formula for alkenes is,
CnH2n
hence, here n = 15
so we get as,
===> C15 H30
identify the most likely cause of earthquakes that occur in the area shown on the map

Answers
The most likely cause of earthquakes that occur in the area shown on the map is due to fault lines in the earth's crust.
What are earthquakes?Earthquakes are natural phenomena characterized by the shaking or trembling of the Earth's surface.
They occur due to the sudden release of energy in the Earth's crust along fault lines, which creates seismic waves that propagate through the Earth.
The Earth's crust is composed of several large tectonic plates that float on the semi-fluid layer of the Earth's mantle.
Learn more about earthquakes at: https://brainly.com/question/248561
#SPJ1
A baker is decorating cupcakes using 3 cherries for every 1 cupcake. If the baker has 12 cherries and 5 cupcakes, what is the theoretical yield?
Answers
Answer:
The baker would only be able to make 4 cupcakes each having 3 cherries on it.
Explanation:
Since the focus is on cherries you can ignore the cupcakes. So if you have 12 cherries and 3 PER cupcake you divide. 12 by 3 to get 4. Even though there will be one cupcake left, they could always give it to me! lol
Hope this helps and have a great day!
Answer:
4
Explanation:
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Which of the following diagrams represents the winter in the southern hemisphere?
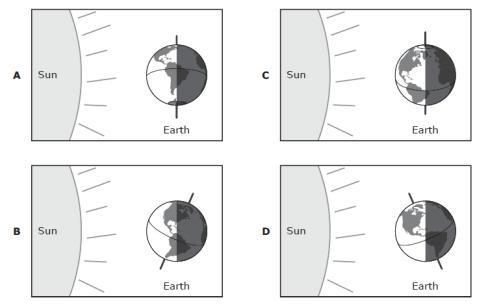
Answers
Answer:
Left position
If this don't help i am sorry but i tried
Explanation:
The figure is representing the motion of revolution of the Earth around the Sun.
This motion lasts approximately 365 days (one year). Since the axis of rotation of the Earth is tilted (by about 23 degrees from the vertical), the amount of light received by different parts of the Earth at the different part of the year is different.
In fact, we observe 4 "extreme" cases:
- Situation at the bottom: here the situation is symmetrical in the two hemispheres, which receive an equal amount of light. So, we have 12 hours of daylight and 12 hours of dark at every point on Earth - this is called equinox, and it occurs in September
- Situation on the right: here we see that the Southern Hemisphere receive much more light than the Northern Hemisphere - so the Southern Hemisphere has more hours of sunlight per day. This is called winter solstice, and it occurs in December - in this case, it is summer in the Southern Hemisphere and winter in the Northern Hemisphere
- Situation at the top: here the situation is symmetrical in the two hemispheres, which receive an equal amount of light. So, we have 12 hours of daylight and 12 hours of dark at every point on Earth - this is called equinox, and it occurs in March
- Situation on the left: here we see that the Northern Hemisphere receive much more light than the Southern Hemisphere - so the Northern Hemisphere has more hours of sunlight per day. This is called summer solstice, and it occurs in June - in this case, it is summer in the Northern Hemisphere and winter in the Southern Hemisphere
how many moles are in 28.65 grams
Answers
Answer:
There are 0.24952533574875027 moles for 28.65 grams.
Explanation:
1 gram = 0.0087094358027487 mole
Lattice energy is an estimate of the bond
conductivity
group
length
strength
Answers
Answer:
Lattice energy is an estimate of the bond strength.
I don't know how to write about tentative.....can u help me?

Answers
Answer:
The definition of tentative is not definite or final. An example of tentative is possible, though not definite, plans to go to the movies sometime on Friday.
Identification and quantification for alcohols dissolved in reagent alcohol
Answers
To identify and quantify alcohols in reagent alcohol, techniques such as gas chromatography (GC), high-performance liquid chromatography (HPLC), spectroscopy (IR and NMR), and refractometry can be employed. These methods separate and detect alcohol compounds based on their properties, providing quantitative data. The choice of technique depends on factors like alcohol nature and desired sensitivity.
To identify and quantify alcohols dissolved in reagent alcohol, you can utilize various analytical techniques. Here are some common methods:
1. Gas Chromatography (GC): GC is a widely used technique for the analysis of alcohols. It involves the separation of alcohol compounds based on their volatility and interaction with the stationary phase. The detector, such as a Flame Ionization Detector (FID), can provide quantitative data.
2. High-Performance Liquid Chromatography (HPLC): HPLC is another powerful technique for alcohol analysis. It involves the separation of alcohol compounds using a liquid mobile phase and a stationary phase. Different detection methods like UV-Vis, refractive index, or mass spectrometry can be used for quantification.
3. Spectroscopic Methods: Various spectroscopic techniques, such as Infrared (IR) spectroscopy and Nuclear Magnetic Resonance (NMR) spectroscopy, can be used for alcohol identification and quantification. IR spectroscopy provides characteristic absorption peaks for functional groups like -OH, while NMR spectroscopy can provide structural information and quantify the concentration of alcohol compounds.
4. Refractometry: Refractometry measures the refractive index of a substance, which can be correlated to the concentration of alcohol in a solution. Calibration curves can be established using known standards for quantification.
It is important to note that the specific method chosen will depend on factors such as the nature of the alcohols, the desired sensitivity, and the available equipment and expertise. Validated reference standards and appropriate sample preparation techniques should be used for accurate identification and quantification of alcohols in reagent alcohol.
To learn more about alcohols refer here:
https://brainly.com/question/29268872#
#SPJ11
Read the article and answer the 6 questions in it.
Answers
Answer:
Yea.....where is the article and the 6 questions?
Answer:
Answering
Explanation:
do u want us to tell u what we got?
A sample of a gas occupies 6.00 liters at a temperature of 200 K. If the pressure remains constant and the temperature is raised to 600 K, the volume of gas sample would be 1.)18.0 L 2.)2.00 L 3.)3.00 L 4.)12.0 L
Answers
Answer:
18
Explanation:
The volume of the gas should be 1. 18.0 L
Calculation of the volume of the gas;Since A sample of a gas occupies 6.00 liters at a temperature of 200 K. If the pressure remains constant and the temperature is raised to 600 K
So here the volume of the gas should be
= 600 * 6 /200
= 18.0 L
hence, the first option is correct.
Learn more about temperature here: https://brainly.com/question/17895578
write a paragraph using the words atom, element, compound, and mixture.
Answers
calculate the theoretical yield for the bromination of both stilbenes
Answers
Answer:
cinnamic acid - 150 mg
cis-stilbene - 100 μL
trans- stilbene - 100 mg
pyridinium tribromide - 200-385 mg
For this data:
moles of cinnamic acid = 0.150 g/148.16 g/mol = 0.001 mols
Theoretical mass of dibromoproduct formed = 0.001 mol x 307.97 g/mol = 0.312 g
cis-stilbene (100 ul = 0.1 ml)
moles of cis-stilbene = 0.1 ml x 1.01 g/mol/180.25 g/mol = 0.00056 mols
Theoretical mass of dibromoproduct formed = 0.00056 mol x 340.05 g/mol = 0.19 g
trans-stilbene
moles of tran-stilbene = 0.1 g/180.25 g/mol = 0.00055 mols
Theoretical mass of dibromoproduct formed = 0.00055 mol x 340.05 g/mol = 0.19 g
Explanation:
What happens to sound waves when you are moving toward the source of the
sound?
A. Wavelength increases.
B. Wave speed decreases.
C. Amplitude decreases.
D. Frequency increases.
Answers
Answer:
B
Explanation:
When 12.0 mL of a 9.08×10−4 M potassium sulfide solution is combined with 22.0 mL of a 6.52×10−4 M zinc bromide solution, does a precipitate form? For these conditions, what is the reaction quotient, Q?
Answers
The reaction quotient Q is 1.08x10⁻¹⁰ when a 2.0 mL of a 9.08×10−4 M potassium sulfide solution is combined with 22.0 mL of a 6.52×10−4 M zinc bromide solution and a precipitate is formed.
The reaction of CoF₂ with NaOH is:
CoF₂(aq) + 2 NaOH(aq) ⇄ Co(OH)₂(s) + 2 NaF(aq).
The solubility product of the precipitate produced which is Co(OH)₂, is as follows:
Co(OH)₂(s) ⇄ Co²⁺(aq) + 2OH⁻(aq)
And Ksp is:
Ksp = 3x10⁻¹⁶= [Co²⁺][OH⁻]²
Molar concentration of both ions is:
Calculation: [Co²⁺] = 0.018Lₓ (8.43x10⁻⁴mol / L) / (0.018 + 0.022)L = 3.79x10⁻⁴M
[OH⁻] = 0.022Lₓ (9.72x10⁻⁴mol / L) / (0.018 + 0.022)L = 5.35x10⁻⁴M
Reaction quotient under these concentrations is:
Q = [3.79x10⁻⁴M] [5.35x10⁻⁴M]²
Q = 1.08x10⁻¹⁰
As Q > Ksp, the equilibrium will shift to the left producing Co(OH)₂(s) the precipitate.
Learn more about reaction quotient:
brainly.com/question/9024475
#SPJ4
a 5.00 g sample of acrylic glass, which is sometimes used as a break-resistant glass substitute, contains 3.57 g of carbon, 0.48 g of hydrogen, and 0.95 g of oxygen. what is the percent composition of acrylic glass?
Answers
The percent composition of acrylic glass is 71.4% carbon, 9.6% hydrogen, and 19.0% oxygen.
What is composition?Composition is the act of combining different elements to create a whole. It is often used in the arts to refer to the arrangement and organization of parts to create a unified work of art. It can also refer to music, literature, photography, and other forms of creative expression. In terms of writing, composition involves crafting logical arguments and choosing words, sentences, and paragraphs carefully in order to convey the desired meaning.
The percent composition of acrylic glass is calculated by taking the mass of each element in the sample and dividing it by the total mass of the sample, then multiplying the result by 100 to get the percentage.
Carbon: 3.57 g / 5.00 g x 100 = 71.4%
Hydrogen: 0.48 g / 5.00 g x 100 = 9.6%
Oxygen: 0.95 g / 5.00 g x 100 = 19.0%
Therefore, the percent composition of acrylic glass is 71.4% carbon, 9.6% hydrogen, and 19.0% oxygen.
To learn more about composition
https://brainly.com/question/26150306
#SPJ4
Help needed fast, please ?

Answers
The standard reduction potential for the half-reaction of Be^2+ + 2e^- -> Be is given as E^0 = 3.83 V.
For the half-cell Hg^2+ | Hg, the standard reduction potential is not provided in the given information. To calculate the electric potential for the voltaic cell, we need the reduction potential for the Hg^2+ | Hg half-cell.
A voltaic cell, also known as a galvanic cell or an electrochemical cell, is an electrochemical device that generates electrical energy through a spontaneous chemical reaction. It consists of two half-cells connected by an external circuit and a salt bridge or porous barrier that allows the flow of ions between the two half-cells.
Each half-cell consists of an electrode immersed in an electrolyte solution. The electrode is typically made of a metal or a conductive material, and the electrolyte is a solution containing ions that can participate in the redox (reduction-oxidation) reaction.
Learn more about electrochemical cell on:
https://brainly.com/question/23631454
#SPJ1
what information can you figure out about an atom using only the atomic number?
Answers
3. Chemical A has a pH value of 9.0. How many times more acidic is chemical B, with a pH value of 8.2, than chemical A? Recall: pH = -log[H]
Answers
The ratio indicates that the hydrogen ion concentration of chemical A is 0.158 times lower than that of chemical B. Alternatively, the hydrogen ion concentration of chemical B is 6.31 times more acidic than that of chemical A.
The pH value of a substance is an essential indicator of its acidity or alkalinity. The pH scale ranges from 0 to 14. The midpoint of the scale is 7.0, which is neutral. Solutions with pH values below 7.0 are acidic, while those with pH values above 7.0 are alkaline.
Acid solutions have a high concentration of hydrogen ions. The negative logarithm of the hydrogen ion concentration (H+) is referred to as the pH. Similarly, solutions with a high hydroxide ion concentration have high pH values. The formula for pH is pH = -log[H].
1. Calculation of [H+] for Chemical A:Hence, we can rearrange the pH equation to calculate the hydrogen ion concentration as follows:[H] = 10^-pH= 10^-9= 1.0 × 10^-9 mol/L2. Calculation of [H+] for Chemical B:pH = -log[H]log[H] = -pHlog[H] = -8.2[H] = 10^-pH[H] = 6.31 × 10^-9 mol/L3.
Calculation of the ratio of [H+] for Chemical A and Chemical B:The ratio of [H+] for chemical A to that of chemical B can be found using the following formula:Ratio = [H+] of Chemical A / [H+] of Chemical B= (1.0 × 10^-9) / (6.31 × 10^-9)= 0.158The ratio indicates that the hydrogen ion concentration of chemical A is 0.158 times lower than that of chemical B. Alternatively, the hydrogen ion concentration of chemical B is 6.31 times more acidic than that of chemical A.
To learn more about hydrogen visit;
https://brainly.com/question/30623765
#SPJ11
Which sentence from the article shows a MAIN problem facing Earth's water supply?
Answers
The sentence that shows a MAIN problem facing Earth's water supply is "We are experiencing the fastest rate of glacial retreat in recorded history."
This sentence highlights the alarming rate at which glaciers, which are a significant source of Earth's freshwater, are melting. The melting of glaciers not only leads to a loss of freshwater but also contributes to rising sea levels, which can have devastating effects on coastal communities. This problem is compounded by the fact that most of Earth's freshwater is locked in glaciers, making it difficult to access and distribute. Additionally, as climate change continues to worsen, the distribution and availability of Earth's water supply may become even more uneven, leading to further challenges in managing this valuable resource. Therefore, it is crucial that we take steps to address the issue of glacial retreat and ensure the sustainability of Earth's water supply for future generations.
learn more about glaciers Refer: https://brainly.com/question/19709729
#SPJ11
complete question :
Which sentence from the article shows a MAIN problem facing Earth's water supply?
A Most of Earth's freshwater is ice, locked in massive glaciers, ice sheets and ice caps.
B Water vapor is not evenly distributed across the atmosphere.
с Residence time for water in the Antarctic ice sheet is about 17,000 years.
D We are experiencing the fastest rate of glacial retreat in recorded history,
A lab is done using different metals and the density of the metals are recorded in a data table. What is the independent variable in the lab?
Answers
Answer: The independent variable is the type of metal being used.
{Note: The "dependent variable" is the "measured density" that corresponds to each of the metals."}.
___________________________________________
Explanation:
___________________________________________
The "independent variable", which is plotted on the "x-axis" (horizontal axis), is the variable that can be "controlled/manipulated". In this case, this would be the type of metal chosen.
The "dependent variable" , which is plotted on the "y-axis" (vertical axis) is the "obtained value/measurement/result" (that "cannot be controlled/manipulated").
In this case, the "density", which is the "measured value" that corresponds to the selected "meal", is the "dependent variable".
___________________________________________
Hope this helpful to you!
Wishing you well!
___________________________________________
1.875mps into kilometers per hour
Answers
Answer:
6,75 kilometres per hour
I need help!!!! please

Answers
6.94 is much more closet to 7.016, so Li 7 is more abundant
How many molecules are in 71.3 moles of H2O
Answers
Use Avogadro’s constant to convert moles into particles.
71.3x6.02x10^23=429.229
There are 429.229 molecules in 71.3 moles of H2O
Which two elements make up this mixture, element C and B element C, and an element D and C or element B And D?
Answers
What is the empirical formula of a compound that is 27.3% carbon, 9.10% hydrogen and 63.6% nitrogen by mass?
Answers
Explanation:
Mass ratio C : H : N = 27.3 : 9.10 : 63.6
Mole ratio C : H : N = 27.3/12: 9.10/1 : 63.6/14
( Divide the mass ratio by their molar masses to find the ratio between moles)
Mole ratio = 2.275 : 9.10 : 4.54
= 2.275/2.275 : 9.10/2.275 : 4.54/2.275
= 1 : 4 : 2
Answer is CH4N2
The empirical formula of a compound is \(CH_4N_2\)
What is an Empirical Formula?The empirical formula of a chemical compound is defined as the simplest whole number ratio of the atoms present in a compound.
The empirical formula of a compound defines the simplest ratio of the number of different atoms present. The molecular formula gives the actual number of each different atom present in a molecule. The molecular formula is mainly used which is a multiple of the empirical formula.
Molecular Formula = n × Empirical Formula
For above given example,
Mass ratio C : H : N = 27.3 : 9.10 : 63.6
Mole ratio C : H : N = 27.3/12: 9.10/1 : 63.6/14
( We divide the mass ratio by their molar masses to find the ratio between moles)
Mole ratio = 2.275 : 9.10 : 4.54 = 2.275/2.275 : 9.10/2.275 : 4.54/2.275
= 1 : 4 : 2
Thus, the empirical formula of a compound is \(CH_4N_2\)
Learn more about Empirical Formula, here:
https://brainly.com/question/14044066
#SPJ5
what are the balanced half-reactions for the electrodes on the left and right? be sure to include states of matter.
Answers
The balanced half-reactions for the electrodes on the left and right are
Ni(s) → Ni²⁺(aq) 2 e⁻ . (left) anode
Cu²⁺(aq) + 2 e⁻ → Cu(s) ( right) cathode
The overall reaction is given as :Ni(s) + Cu²⁺(aq) → Ni²⁺(aq) + Cu(s)
A voltaic cell is constructed with a Cu/Cu²⁺ half-cell and an Ni/Ni²⁺ half-cell. The nickel electrode is negative (anode) and the copper electrode is positive (cathode). In the anode takes place the oxidation and in the cathode takes place the reduction. The corresponding half-reactions are:
Anode (oxidation): Ni(s) → Ni²⁺(aq) 2 e⁻
Cathode (reduction): Cu²⁺(aq) + 2 e⁻ → Cu(s)
The overall reaction is:
Ni(s) + Cu²⁺(aq) → Ni²⁺(aq) + Cu(s)
To know more about half reactions here
https://brainly.com/question/28380657
#SPJ4
The complete question is
A voltaic cell is constructed with a Cu/Cu2 half-cell and an Ni/Ni2 half-cell. what are the balanced half-reactions for the electrodes on the left and right? be sure to include states of matter.