For aluminum, Al, the heat of fusion at its normal melting point of 660 °C is 10.8 kJ/mol.
The entropy change when 1.74 moles of solid Al melts at 660 °C, 1 atm is __J/K.
Answers
The entropy change when 1.74 moles of solid Al melts at 660 °C and 1 atm is approximately 6.39 J/K.
To calculate the entropy change when solid aluminum (Al) melts at 660 °C and 1 atm, we need to use the equation:
ΔS = ΔH_fus / T
where ΔS is the entropy change, ΔH_fus is the heat of fusion, and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
T = 660 °C + 273.15 = 933.15 K
Next, we can substitute the values into the equation:
ΔS = (10.8 kJ/mol) / (1.74 mol) / (933.15 K)
Now, let's perform the calculation:
ΔS = 10.8 kJ / 1.74 mol / 933.15 K = 6.39 J/K
The entropy change is a measure of the disorder or randomness in a system. When a solid substance melts, the particles gain more freedom of movement, leading to an increase in entropy. In this case, the value of 6.39 J/K indicates an increase in disorder during the melting process of aluminum.
For such more questions on entropy
https://brainly.com/question/30704498
#SPJ8
Related Questions
Which term represent a phase change ?
Burning
Melting
Expanding
Cutting
Answers
We went over this in school earlier in the year.
Hope this helps you have a great day
Good luck
HURRRRRRY PLEASEEEE HELP!!!!!!
What type of plate boundary are the arrows on the image showing?

Answers
Answer:
Transform boundaries because they're sliding past each other.
I hope this helped!
an endothermic animal is warm blooded. This means it can do what
Answers
Answer:
Endothermic animals are those that must generate their own heat to maintain an optimal body temperature.
Explanation:
Answer:
There
Explanation:
Endothermic animals are those that must generate their own heat to maintain an optimal body temperature. In ordinary language, these animals are commonly referred to as "warm-blooded." The term endotherm comes from the Greek endon, meaning within, and thermos, which means heat
Which of the following statements about enzymes is NOT true? *
1.All enzymes have the same shape as their substrates.
2.Enzymes are proteins.
3.The shape of an enzyme allows it to do its job.
Answers
a.
Suppose a group of bobcats that normally live and hunt in the tops of mountains, start hunting prey at the base of the
mountain and move into the valley below. Which of the following will most likely happen to the prey in the valley?
Their numbers will soon increase.
b. Their numbers will soon decrease.
C. They will move to the tops of the mountains.
d. They will remain unchanged.
Please select the best answer from the choices provided
Ο Α
OB
C
OD
Mark this and retum
Save and Exit
Next
Submit
Answers
Answer:
University of University of South New York Jersey has is a the best in the world the most important most important aspect of aspect of life is life and marriage is in my a lot more of a urban
what atom does not contain neutron
Answers
Answer:
There is only one stable atom that does not have neutrons. It is an isotope of the element hydrogen called protium. Protium, which contains a single proton and a single electron, is the simplest atom. All other stable atoms contain some number of neutrons. hope this helps you :)
There is only one stable atom that does not have neutrons. It is an isotope of the element hydrogen called protium. Protium, which contains a single proton and a single electron, is the simplest atom. in size which cannot accommodate any heavier neutron. It also makes hydrogen atom unstable in nature. All elements have atoms with neutrons except for one. A normal hydrogen (H) atom does not have any neutrons in its tiny nucleus. That tiny little atom (the tiniest of all) has only one electron and one proton. ... Deuterium is a hydrogen atom with an extra neutron and tritium has two extra. Neutron stars are made out of neutrons, so there's definitely no atoms there. The sparse matter which is between stars or between galaxies, which is the majority of the mass in the universe (!) (not including dark matter), is also not mostly made of atoms. It's apparently a plasma-like mix of protons and electrons. Neutron, neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1,839 times greater than that of the electron. An atom is the smallest building block or fraction of an accessible chemical element that retains the chemical properties of that element. The origin of the English word goes back to the Greek word atomos, which means indivisible; It was believed that nothing is smaller than an atom.
Can someone help me Please?
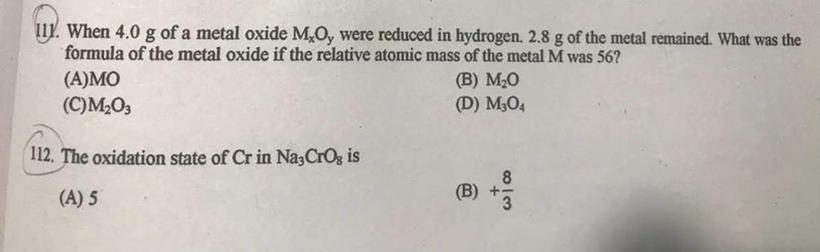
Answers
Answer:
111. C . M2O3
112. A. 5
What amount of heat is required to increase the temperature of 75.0 g of water from 22.3˚C to 36.1˚C?
Answers
Answer:
4332.5 j
Explanation:
Specific heat of water is 4.186 j /g-C
4.186 j / g-C * 75 g * (36.1-22.3 C) =
48g of methane was burned in an excess of air. What mass of carbon dioxide would be produced in the reaction assuming complete combustion? Use the information below to answer the question.

Answers
Answer:
132g
Explanation:
CH4= 12+4=16
moles= m/Mr moles=48/16=3 mol
CO2= Mr=44
there are the same number of moles as methane:
Mass= Mrxmoles
44x3=132g
The mass of the carbon dioxide produced by the combustion of the 48 g of methane is equal to 132 g.
What is the combustion reaction?Combustion reaction involves the complete oxidation of the hydrocarbon such reactions generally release only carbon dioxide and water as products.
A hydrocarbon undergoes combustion to produce carbon dioxide and water as the product. No other side products are formed in the combustion reaction.
The given combustion reaction of the methane can be written as follows:
CH₄ + 2 O₂ → CO₂ + 2H₂O
Given the amount of methane for combustion = 48 g
The molar mass of methane = 16g/mol
The molar mass of the carbon dioxide, CO₂ = 44g/mol
16 grams of methane produce carbon dioxide = 44g
The 48 grams of methane will produce CO₂ = (44/16) ×48 = 132 g
Therefore, the mass of 132 g of carbon dioxide is produced by the combustion of 48g of methane.
Learn more about combustion reactions, here:
brainly.com/question/12172040
#SPJ2
What type of energy is stored in the nucleus of an atom
Answers
Answer:
nuclear energy
Explanation:
this holds the nucleus together
Brainpop bacteria True or false
Answers
Brainpop is an educational platform that offers animated videos and interactive quizzes on various subjects, including science, history, and mathematics. One of the topics covered on Brainpop is bacteria.
True Brainpop offers information on bacteria.
The answer to this question is true. Brainpop has a section dedicated to bacteria, where students can learn about different types of bacteria, their characteristics, and their role in the environment and human health.
Through animated videos, students can learn about the structure of bacteria, how they reproduce, and how they play a role in nutrient cycling. The platform also provides interactive quizzes and activities to test students' knowledge on the topic.
Overall, Brainpop is a useful tool for students to learn about bacteria and other scientific concepts in an engaging and interactive way.
For more such question on bacteria
https://brainly.com/question/30307005
#SPJ11
Note- The Question seems Incomplete, and complete question isn't available in the search engine.
A 10.0 L balloon contains helium gas at a pressure of 655 mmHg. What is the final pressure, in millimeters of mercury, if the final volume is 13.8 L? Please show your work in order to receive credit.
Answers
Answer:
474. 64 mm Hg
Explanation:
P1 = 655 mm Hg V1 = 10 LP2 = ? V2 = 13. 8 Lusing Boyle's Law that is
P1. V1 = P2. V2
655 × 10 = P2 × 13. 8
\(P2 = \frac{655 \times 10}{13.8} \)
P2 = 474. 64 mm Hg
A solution is prepared by mixing 50.0 mL of 0.600 M Sr(NO3)2 with 50.0 mL of 1.60 M KIO3. Calculate the equilibrium Sr2+ concentration in mol/L for this solution. Ksp for Sr(IO3)2 = 2.30E-13.
Answers
The equilibrium concentration in mol/L for Sr₂+ ions with Ksp value Sr(IO3)2 = 2.30E-13 is 7.04E-9 M.
The balanced chemical equation for the reaction that occurs between Sr(NO₃)₂ and KIO₃ is:
Sr(NO₃)₂ + 2 KIO₃ → Sr(IO₃)₂ + 2 KNO₃
Using the stoichiometry of the balanced equation, we can see that for every 1 mole of Sr(NO₃)₂ that reacts, 1 mole of Sr(IO₃)₂ is formed. Therefore, the initial concentration of Sr₂+ ions is 0.600 M, and the concentration of IO₃- ions is 2 × 1.60 M = 3.20 M (because 2 moles of KIO₃ are used for every mole of Sr(NO₃)₂).
The solubility product expression for Sr(IO₃)₂ is:
Ksp = [Sr₂+][IO₃-]²
At equilibrium, the concentration of Sr₂+ ions will be x (in mol/L), and the concentration of IO₃- ions will be 3.20 - 2x (in mol/L) because 2 moles of IO₃- are used for every mole of Sr(IO₃)₂ that forms. The concentration of NO3- ions can be ignored because they are spectator ions and do not participate in the equilibrium.
Substituting these concentrations into the Ksp expression gives:
2.30E-13 = x(3.20 - 2x)²
Solving this equation for x gives:
x = 7.04E-9 M
Therefore, the equilibrium concentration of Sr₂+ ions is 7.04E-9 M.
To learn more about equilibrium concentration here
https://brainly.com/question/32070728
#SPJ4
Explain how you would calculate how many seconds old you are. What conversion factors would you use?
Answers
365 days = 1 years
1 day = 24 hours
1 hour = 60 minutes
1 minute = seconds
I would be able to solve it.
Convert .059 Hm into cm.
Answers
Answer:
One Hectometer is 10,000 centimeters. So multiply your hectometer by 10,000, and that's your conversion. Your answer is 590.
What is the coefficient for water molecules in the balanced version of the following redox reaction? cr2o2−7 c2h4o→c2h4o2 cr3
Answers
The given redox reaction is:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+
To balance this reaction, we first balance the oxygen atoms by adding H2O on the right side of the equation. The number of H2O molecules added depends on the number of oxygen atoms needed. In this case, we need three O atoms on the right side, so we add three H2O molecules to the right side of the equation:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+ + 3H2O
Next, we balance the hydrogen atoms by adding H+ ions on the left side of the equation. The number of H+ ions added depends on the number of hydrogen atoms needed. In this case, we need eight H atoms on the left side, so we add eight H+ ions to the left side of the equation:
Cr2O7^2- + C2H4O + 8H+ → C2H4O2 + Cr3+ + 3H2O
Finally, we balance the charge by adding electrons. The number of electrons added depends on the difference in charge on the left and right side of the equation. In this case, the left side has a charge of -2 (from the Cr2O7^2- ion), while the right side has a charge of +3 (from the Cr3+ ion). This means that we need to add 5 electrons to the left side of the equation to balance the charge:
Cr2O7^2- + C2H4O + 8H+ + 5e- → C2H4O2 + Cr3+ + 3H2O
Therefore, the coefficient for water molecules in the balanced version of the given redox reaction is 3.
To know more about redox reaction visit
https://brainly.com/question/28300253?
#SPJ11
2. Which equation is correctly balanced?
A. H₂+O₂ → H₂O
B.
Ca + Cl₂ → CaCl
C.
2H2 + O2 → 2H₂O
D.
Ca + Cl₂ → Ca₂Cl
Answers
The equation is correctly balanced is option C is correct 2H₂ + O₂ → 2H₂O
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation
Here in the given reaction option C is correct because 4 atoms of hydrogen on reactant side. 2 atoms of oxygen on reactant side
2H₂ + O₂ → 2H₂O
And the other reaction is not balanced reaction because in reactant side or in product side there is difference so it is not balanced
Know more about balanced equation
https://brainly.com/question/12192253
#SPJ1
(04.04 LC)
During light-dependent reactions, carbon dioxide is converted to glucose.
True
O False
Answers
Answer:
false
Explanation:
it happens in light-independent reactions
HELP PLEASE!!!
How are both stability and change seen in properties of elements?
Answers
Answer:
In both natural and built systems, stability and change are an important focus of study for both scientists and engineers. Stability refers to a system that is unchanging. ... A dynamic equilibrium exists when chemical reactions or physical movements occur at rates that balance out, creating no net change in a system.
Answer:
In both natural and built systems, stability and change are an important focus of study for both scientists and engineers. Stability refers to a system that is unchanging. ... A dynamic equilibrium exists when chemical reactions or physical movements occur at rates that balance out, creating no net change in a system.
Explanation:
g sio2 is a(n) covalent network solid. ki is a(n) -- solid. ti is a(n) -- solid. c6h12o6 is a(n) -- solid.
Answers
The kinds of the solids are;
SiO2 - Covalent network solid
C6H12O6 - Covalent solid
KI - Ionic solid
What is a covalent network solid?
A covalent network solid, often referred to as a network covalent solid or just a network solid, is a category of solid material in which the atoms that make up the material are strongly covalently linked to one another, forming an extended three-dimensional network structure.
Covalent network solids are kept together by a dense network of covalent bonds, as opposed to molecular or ionic solids, which are held together by weaker intermolecular forces or ionic interactions, respectively.
Learn more about covalent network solid:https://brainly.com/question/30458552
#SPJ4
The equation shows a reaction at equilibrium.
3H2 (g) + N, (8) = 2NH3 (g) + 92 kJ
Which of the following describes what happens if NH is added to the system?
Equilibrium is restored as the reaction shifts toward the reactants
Equilibrium is restored as the reaction shifts toward the products.
Equilibrium is restored as more heat energy is released from the system.
Equilibrium is restored as the concentration is increased to the products
Answers
If NH is introduced to the system, equilibrium is restored as the reaction moves towards the reactants. Option A is correct.
According to Le Chatelier's principle, if a stress is applied to a system at equilibrium, the system will adjust in a way that partially offsets the effect of the stress and restores equilibrium. The reaction:
3H₂ (g) + N₂ (g) ⇌ 2NH₃ (g) + 92 kJ
In this case, adding NH₃ to the system would increase the concentration of products, causing the equilibrium to shift towards the reactants in order to reduce the excess products.
This means that the forward reaction (production of NH₃) would slow down, while the reverse reaction (breakdown of NH₃) would speed up until equilibrium is re-established. Since the forward reaction is exothermic (heat-releasing), increasing the concentration of reactants by shifting the equilibrium towards the left would also decrease the heat energy in the system, helping to partially offset the addition of NH₃. Option A is correct.
To know more about the Equilibrium, here
https://brainly.com/question/30101313
#SPJ1
When mixed, aqueous solutions of aluminum nitrate, Al(NO3)3, and ammonium carbonate, (NH4)2CO3, will form a precipitate of aluminum carbonate, Al2(CO3)3. The balanced equation is:
2Al(NO3)3(aq) + 3(NH4)2CO3(aq) à Al2(CO3)3(s) + 6NH4NO3(aq)
Which of the following statements regarding this reaction is incorrect?
A)2 moles of Al(NO3)3 will react with 3 moles of (NH4)2CO3.
B)If 6 moles of (NH4)2CO3 react with sufficient Al(NO3)3, 2 moles of Al2(CO3)3 will be formed.
C)If 0.5 mole of (NH4)2CO3 react with sufficient Al(NO3)3, 3 moles of Al2(CO3)3 will be formed.
D)If 1.5 moles of Al2(CO3)3 are formed, given sufficient starting materials, then 9 moles of NH4NO3 will also be formed.
E)4 moles of Al(NO3)3 will react with 6 moles of (NH4)2CO3.
F)If 4 moles of Al(NO3)3 reacts with 9 moles of (NH4)2CO3 there will be left over (NH4)2CO3
Answers
The incorrect statement regarding the reaction between aqueous solutions of aluminum nitrate, Al(NO3)3, and ammonium carbonate, (NH4)2CO3, which form a precipitate of aluminum carbonate, Al2(CO3)3 is If 0.5 mole of (NH4)2CO3 react with sufficient Al(NO3)3, 3 moles of Al2(CO3)3 will be formed. Option C.
A chemical equation is a description of the chemical reaction that takes place. It contains the formulae of the reactants and products separated by an arrow. The arrow indicates the direction of the reaction. The stoichiometric coefficients in the equation represent the number of moles of each substance involved in the reaction.
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It's important to keep in mind that the law of conservation of mass applies to chemical reactions. This means that the number of atoms of each element present in the reactants must equal the number of atoms of that element present in the products. Thus, stoichiometry plays a significant role in determining how much product is formed from a given amount of reactant and vice versa. Option C.
More on aqueous solutions: https://brainly.com/question/31271307
#SPJ11
A 96,000 gallon pool has a free chlorine level of 1. 4 ppm and a total chlorine level of 1. 8. It takes 2 ounces of dry chlorine (at 67%) to raise a 10,000 gallon pool's chlorine level 1 ppm. How much chlorine is needed to reach break point chlorination? Show all work
Answers
To reach break point chlorination in a 96,000 gallon pool with a difference of 0.4 ppm between the free chlorine and total chlorine levels, approximately 7.68 ounces of chlorine is needed.
To calculate the amount of chlorine needed to reach break point chlorination in a 96,000 gallon pool, we first need to find the difference between the total chlorine and free chlorine levels. Break point chlorination is achieved when the free chlorine level equals the total chlorine level.
Given that the free chlorine level is 1.4 ppm and the total chlorine level is 1.8 ppm, the difference between them is:
1.8 ppm - 1.4 ppm = 0.4 ppm
Now, we need to determine the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool. The given information states that it takes 2 ounces of dry chlorine (67% concentration) to raise a 10,000 gallon pool's chlorine level by 1 ppm.
To calculate the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool, we can set up a proportion:
2 ounces / 1 ppm = X ounces / 0.4 ppm
Solving for X (the amount of chlorine needed for 0.4 ppm increase in a 10,000 gallon pool):
X = (2 ounces / 1 ppm) * 0.4 ppm = 0.8 ounces
Now, we can calculate the amount of chlorine needed for the 96,000 gallon pool by scaling the chlorine required for the 10,000 gallon pool:
Amount of chlorine needed = (0.8 ounces / 10,000 gallons) * 96,000 gallons
Amount of chlorine needed = 0.8 ounces * 9.6 = 7.68 ounces
Therefore, approximately 7.68 ounces of chlorine is needed to reach break point chlorination in the 96,000 gallon pool.
For more such question on chlorination. visit :
https://brainly.com/question/24218286
#SPJ8
What special label do you have to use when naming ionic compounds with transition metals?
A) Roman Numerals
B) Greek Numerals
Answers
Naming Ionic compounds with transition metals requires the use of a roman numeral. The charge of the metal ion must be written in the name of the compound with a roman numeral. This is because transition metals can have more than one valence (or charge).
government entity sets a Food Defect Action Level (FDAL) for the various foreign substances that inevitably end up in the foods we eat. The FDAL level for insect filth in peanut butter is 0.5 insect fragment (larvae, eggs, body parts, and so on) per gram. Suppose that a supply of peanut butter contains 0.5 insect fragment per gram. Compute the probability that the number of insect fragments in a 4-gram sample of peanut butter is (a) exactly three. Interpret the results. (b) fewer than three. Interpret the results. (c) at least three. Interpret the results. (d) at least one. Interpret the results. (e) Would it be unusual for a 4-gram sample of this supply of peanut butter to contain five or more insect fragments?
Answers
a. Probability (X = 3) = 0.180
b. Probability(X < 3) = 0.676
c. Probability(X >= 3) = 0.324
d. Probability (X >= 1) = 0.865
e. Probability (X >= 5) = 0.0525
How do we calculate?(a) we find the Probability of exactly three insect fragments in a 4-gram sample as :
λ = 0.5 * 4 = 2
P(X = 3) = (e^(-2) * 2^3) / 3!
P(X = 3) = 0.180
(b) Probability of fewer than three insect fragments in a 4-gram sample:
P(X < 3) = P(X = 0) + P(X = 1) + P(X = 2)
P(X < 3) = e^(-2) + (e^(-2) * 2) + (e^(-2) * 2^2)
P(X < 3) = 0.676
(c) Probability of at least three insect fragments in a 4-gram sample:
P(X >= 3) = 1 - P(X < 3)
P(X >= 3) ≈ 1 - 0.676
P(X >= 3) = 0.324
(d) Probability of at least one insect fragment in a 4-gram sample:
P(X >= 1) = 1 - P(X = 0)
P(X >= 1) ≈ 1 - e^(-2)
P(X >= 1) = 0.865
e. The Unusualness of containing five or more insect fragments is found as :
P(X >= 5) = 1 - (P(X = 0) + P(X = 1) + P(X = 2) + P(X = 3) + P(X = 4))
P(X >= 5) = 1 - (0.1353 + 0.2707 + 0.2707 + 0.1805 + 0.0903)
P(X >= 5) ≈ 1 - 0.9475
P(X >= 5) = 0.0525
In conclusion, the probability of a 4-gram sample of this supply of peanut butter containing five or more insect fragments is found to be 0.0525.
Learn more about probability at:
https://brainly.com/question/24756209
#SPJ4
Scientist use alternative ways to seek out if a fault is active which one isn't include
Answers
Answer:
Geologists commonly consider faults to be active if there has been movement observed or evidence of seismic activity during the last 10,000 years. Active faulting is considered to be a geologic hazard - one related to earthquakes as a cause.
Explanation:
Plz mark brainliest thanks
Please help with (i) (j) (k)

Answers
Answer:
Explanation: l m n o p q r s t u v w x y z
Suppoe that you ue 4. 25 g of Iron in the chemical reaction: 2Fe() 3Cu2(aq) rightward arrow 2Fe3(aq) 3Cu(). What i the theoretical yield of Cu(), in gram?
Answers
Theoretical yield of copper is 7.22 g when 4.25g of iron in the chemical reaction.
2Fe(s) + 3Cu2(aq.) ----> 2Fe3(aq.) + 3Cu (s)
Molar mass of Iron (Fe) is 56g/mole.
Molar mass of copper (cu) is 63.5g/mole.
2 mole ( 2 * 56g/mole) Iron produces = 3 mole ( 3 * 63.5g/mole ) copper
Theoretical yield is the maximum amount of product that could be formed from the given amounts of reactants. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. The theoretical yield is the amount of product that would be formed from a reaction if it was 100% efficient.
There are 4. 25 g of Iron .so,
4.25 g Iron produces = (3 * 63.5g / 2 * 56 g ) * 4.25 g copper
= 7.22 g copper
Theoretical yield of copper is 7.22g.
To learn more about Theoretical yield please visit:
https://brainly.com/question/2765357
#SPJ4
Which is the electron configuration for boron?
1s²2s³
1s²2s²3s¹
1s¹2s²2p²
1s²2s²2p¹
Answers
1s²2s²2p¹.
Explanation:
The electronic configuration of Boron is 1s²2s²2p¹ .
The electron configuration for boron is 1s²2s²2p¹.
What is electron configuration?Electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.
Electron configuration can be written according to the following rules-
1. Aufbau Principle
2. Pauli's exclusion principle
3. Hund's rule
Electrons will be filled according to the increasing order of the energy as per the n+l rule. Boron has atomic number as 5 that means it has 5 electrons.
Therefore, The electron configuration for boron is 1s²2s²2p¹.
To learn more about Electron configuration click:
https://brainly.com/question/29757010
#SPJ5
What is the difference between heat transfer and heat transformation
Answers
Answer:
heat transfer is when you transfer heat from one thing to another and heat transformation is when heat is transforming into something else hope it helps
Explanation: