For the following reaction, 0.267 moles of carbon dioxide are mixed with 0.263 moles ofpotassium hydroxide.
What is the formula for the limiting reagent?
What is the maximum amount of potassium carbonate that can be produced?
moles
Answers
It is clear from the equation above what happens when one mole of carbon burns in air.
then 44g [Molecular Mass of Carbon Dioxide] of carbon dioxide are created. (ii) In the equation above, 44g of carbon dioxide is created when 32g of oxygen react with 1 mole of carbon. Similar to this, you should divide the weight of the oxygen molecule by its molecular weight. After calculating, we discover that there are 0.5 moles of oxygen in each process. Due to the availability of both and their equal proportions, none of the reactants functions as a limiting reactant. They can be combined in one way to create the well-known molecule carbon dioxide. For each sample of carbon dioxide, 32.0g of oxygen are present. Carbon 12.0 g. This can be simplified to a mass ratio of 2.66 to 1 for oxygen and carbon by dividing 32.0 by 12.0. We only add up the contributions made by each element or atom to determine the molar mass. One carbon atom contributes 12.01 g/mol to carbon dioxide (CO2), while the two oxygen atoms together contribute (2)(16.00) = 32.00 g/mol. Therefore, 12.01 + 32.00 = 44.01 g/mol is the molar mass.
To learn more about molecular please click on below link
https://brainly.com/question/14614762
#SPJ4
Related Questions
1. Pressure of a gas (decreases, increases) if the volume decreases when temperature
is constant.
Answers
Answer:
If temperature is held constant, the equation is reduced to Boyle's law. Therefore, if you decrease the pressure of a fixed amount of gas, its volume will increase. ... Gay-Lussac's law states that at constant volume, the pressure and temperature of a gas are directly proportional.
Explanation:
i hope this helped
Please helppppppppppppppppppp
50 ppoints

Answers
Answer:
I can’t see the whole page
Explanation:
Carbon disulfide and carbon monoxide are produced when carbon is heated with sulfur dioxide.
5C(s)+2SO2(g)→CS2(l)+4CO(g)
How many moles of C are needed to react with 0.460 mole SO2?
How many moles of CO are produced when 2.0 moles C reacts?
How many moles of SO2 are required to produce 0.35 mole CS2?
How many moles of CS2 are produced when 2.4 moles C reacts?
Answers
1) To react with 0.460 mole of SO₂, 1.15 moles of C are needed.
2) When 2.0 moles of C reacts, 1.6 moles of CO are produced.
3) To produce 0.35 mole of CS₂, 0.70 moles of SO₂ are required.
4) When 2.4 moles of C reacts, 0.48 moles of CS₂ are produced.
1.
From the balanced equation, the stoichiometric ratio between C and SO₂ is 5:2. Therefore, to calculate the moles of C required, we can set up a proportion:
(5 moles C / 2 moles SO₂) = (x moles C / 0.460 moles SO₂)
Solving for x, we find:
x = (5/2) × 0.460 = 1.15 moles C
2.
From the balanced equation, the stoichiometric ratio between C and CO is 5:4. Therefore, to calculate the moles of CO produced, we can set up a proportion:
(5 moles C / 4 moles CO) = (2.0 moles C / x moles CO)
Solving for x, we find:
x = (4/5) × 2.0 = 1.6 moles CO
3.
From the balanced equation, the stoichiometric ratio between SO₂ and CS₂ is 2:1. Therefore, to calculate the moles of SO₂ required, we can set up a proportion:
(2 moles SO₂ / 1 mole CS₂) = (x moles SO₂ / 0.35 moles CS₂)
Solving for x, we find:
x = (2/1) × 0.35 = 0.70 moles SO₂
4.
From the balanced equation, the stoichiometric ratio between C and CS₂ is 5:1. Therefore, to calculate the moles of CS₂ produced, we can set up a proportion:
(5 moles C / 1 mole CS₂) = (2.4 moles C / x moles CS₂)
Solving for x, we find:
x = (1/5) × 2.4 = 0.48 moles CS₂
Learn more about number of moles from the link given below.
https://brainly.com/question/20370047
#SPJ4
For 2NaOH + H2SO4 --> Na2SO4 + 2H2O
given 10 moles of NaOH, find how many moles of Na2504 will be made
Answers
In the balanced chemical equation 2NaOH + \(H2SO4\) → \(Na2SO4\) + \(2H2O\), the stoichiometric ratio between NaOH and \(Na2SO4\) is 2:1. This means that for every 2 moles of NaOH reacted, 1 mole of \(Na2SO4\) is produced. Given that we have 10 moles of NaOH, we can calculate the moles of \(Na2SO4\) using stoichiometry.
To find the moles of \(Na2SO4\), we divide the moles of NaOH by the stoichiometric coefficient ratio. In this case, it would be:
10 moles NaOH / 2 = 5 moles \(Na2SO4\).
Therefore, 10 moles of NaOH will produce 5 moles of \(Na2SO4\).
In summary, if we start with 10 moles of NaOH and react it according to the balanced equation, we will produce 5 moles of \(Na2SO4\). This calculation is based on the stoichiometric ratio between NaOH and \(Na2SO4\) in the chemical equation.
Learn more about balanced chemical equation here:
https://brainly.com/question/14072552
#SPJ11
any help would be much appreciated, i'm being timed :( i'll mark brainliest and 5 star

Answers
Answer:
GROUP 16
Explanation:
Answer:
group 2
Explanation:
50 POINTS?!!!!!!
if you measure 0.0356 g of mg for your first trial, how many moles of hydrochloric acid would be needed to react with it? the molar mass of mg is 24.31 g/mol
Answers
0.00292 mol of HCL would be needed to react with 0.0356 g of Mg.
Mass of mg = 0.0356 g
Molar mass of mg is 24.31 g/mol
moles of mg= 0.0356/24.31
=0.00146moles
Mg+2HCL→MgCl₂ +H₂
From above equation , 1 mol of mg requires 2 mol of HCL
∴ 0.00146 mol of Mg will require =2×0.00146 mol of HCL
=0.00292 mol of HCL.
#SPJ4
What is the molality of a solution?
A. The number of grams of a solute per liter of solution
B. The number of moles of a solute per kilogram of solution
C. The number of grams of a solute per mole of solvent
D. The number of moles of a solute per kilogram of solvent
Answers
Explanation: The amount of a material in a given volume of solution is measured in molarity (M). The moles of a solute per liter of a solution is known as molarity. The molar concentration of a solution is also known as molarity.
Hope that helps! Have a good day :)
Answer: The best answer is D. The number of moles of a solute per kilogram of solvent
Explanation:
I hope this helps, whereas, the molality doesn't represent moles nor liters.
What is the difference between criminology and forensic science?
Criminology analyzes how crime affects the community or society as a whole, while forensic science analyzes individual crimes and evidence
O Criminology is an applied Science, while forensic science is an academic science
O Criminologists work closely with law enforcement, while forensic scientists work with academic institutions
o Criminology is a type of forensic science
Answers
Answer:
pick the second one I've done that test
Answer:
Criminology analyzes how crime affects the community or society as a whole , while forensic science analyzes individual crimes and evidence.
There is a Canvas AUDIO tutorial available for this problem. Neglecting activities, calculate the pH of a solution containing 0.018 M NaOH plus 0.0120 M LINO3. 4.0 12.26 Using activities, re-calculate the actually pH of the solution. 4.9 12.2 X Check the number of significant figures. More Information
Answers
The pH of the solution, neglecting activities, is approximately 12.26.
To calculate the pH of the solution, we need to consider the dissociation of water, as well as the dissociation of the solutes NaOH and LiNO3. Let's go through the steps:
Step 1: Identify the ions present in the solution:
The solution contains NaOH and LiNO3. NaOH dissociates into Na+ and OH- ions, while LiNO3 dissociates into Li+ and NO3- ions.
Step 2: Write the balanced chemical equations for the dissociation of NaOH and LiNO3:
NaOH dissociates as follows:
NaOH -> Na+ + OH-
LiNO3 dissociates as follows:
LiNO3 -> Li+ + NO3-
Step 3: Calculate the concentration of each ion:
Given that the concentration of NaOH is 0.018 M and LiNO3 is 0.0120 M, the concentration of Na+ and Li+ ions is equal to their respective initial concentrations.
For NaOH:
[Na+] = 0.018 M
[OH-] = 0.018 M (since NaOH dissociates in a 1:1 ratio)
For LiNO3:
[Li+] = 0.0120 M
[NO3-] = 0.0120 M (since LiNO3 dissociates in a 1:1 ratio)
Step 4: Calculate the concentration of H+ ions:
Since we have the concentrations of OH- ions, we can use the fact that water undergoes auto-ionization to determine the concentration of H+ ions.
[H+] = Kw / [OH-]
At 25°C, the value of Kw (the ion product of water) is approximately 1.0 x 10^-14.
[H+] = (1.0 x 10^-14) / (0.018)
Using a calculator, we find that the concentration of H+ ions is approximately 5.56 x 10^-13 M.
Step 5: Calculate the pH:
pH = -log[H+]
pH = -log(5.56 x 10^-13)
Using a calculator, we find that the pH of the solution neglecting activities is approximately 12.26.
Now, let's consider the re-calculation of pH using activities:
To re-calculate the pH using activities, we need additional information, such as activity coefficients for the ions present. However, the given question does not provide the necessary activity coefficients or any other information required for activity calculations. Therefore, it is not possible to re-calculate the actual pH of the solution using activities without that information.
In summary:
The actual pH of the solution considering activities cannot be determined without additional information, such as activity coefficients.
Learn more about ph here:
https://brainly.com/question/30761746
#SJP11
Match the landform to its description.
caldera
made of pieces of lava
volcanic soil
material fills in valleys
cinder cone
bowl-shaped depression
shield volcano
wide summit, gentle slope
lava plateau
rich in nutrients
Answers
Answer:
cinder cone: made of pieces of lava
shield volcano: wide summit, gentle slope
volcanic soil: rich in nutrients
caldera: bowl shaped depression
lava plateau: material fills in valleys
Explanation:
it’s right
Answer:
Caldera, Bowl-Shaped depression
Shield volcano, Wide summit, Gentle slope
Lava plateau, Material fills in valleys
Volcanic soil, Rich nutrients
Cinder Cone, Made of pieces of lava
Explanation:
Got it right on edge
matter graphic organizer
Answers
_____energy tells us how fast the reaction willproceed.
Answers
Explanation
Kinetic energy is a modality of energy present in all moving bodies. According to the SI, its unit of measurement is the joule. Furthermore, this energy is a scalar quantity that has exclusively positive values.
Kinetic energy is proportional to the square of the body's velocity. Thus, if the speed of a body double, its kinetic energy will increase four times, if the speed of a body triple, then this increase will be nine times.
Answer
Kinect energy tells us how fast the reaction will proceed.
All of the following are nutrients EXCEPT:
a. fats
b. water
c. protein
d. blood
HURRY UP AND HELP ME
Answers
Answer:
\(d.blood\)
Explanation:
Hope it helps!!!
Hope this answer helps
heeeeeeeeeeeeeeelpheeeeeeeeeeeeeeelp

Answers
Answer:
the mirror forms a virtual inlarged image
Explanation:
What's the chemical reaction of Grignard in Organic Chemistry?
Answers
Answer:
Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. They're extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds
Explanation:
hope this helps!
Which best describes what is made of matter?
all living things and objects
all atoms
all solid objects and atoms
all living things
Answers
Answer:
well all living things made up of objects
Explanation:
Hope this helps:)
All living things and objects best describe matter.
What is matter?Matter is a substance which is made up of various types of particles which occupy space and have inertia . All living things and objects are made up of various types of particles that occupy space and have inertia .
Depending on temperature and other factors matter is able to exist in different phases. Most common of which are solid, liquid and gas. Matter can exist in more than one state depending on the temperature and pressure .
State of matter can be changed by heating or cooling and even by changing the applied pressure.When a state changes matter does not break rather its state changes though its chemical composition remains same.
Physical characteristics of matter are shape, color, size and temperature. Every matter is made up of elements which cannot be broken down further by ordinary chemical reactions.
Learn more about matter, here:
https://brainly.com/question/12972782
#SPJ5
Can anyone please suggest me a good and easy project topic for physics and chemistry. For class nine or ten students.Please please please help me.
Answers
URGENT!! ILL GIVE
BRAINLIEST! AND 100 POINTS

Answers
The best explanation for the phenomenon of a prism dispersing white light into a spectrum is that different frequencies of visible light move at different speeds inside the prism. This is known as the process of refraction.
The correct option is D.
What is dispersion of light?Dispersion of light is the separation of white light into its component colors.
When white light enters a prism, the different colors (wavelengths) that make up white light refract, or bend, at slightly different angles as they pass through the prism.
This is due to the fact that different colors of light have different refractive indices or the degree to which light bends as it passes through a medium. This means that each color of light is separated, forming a spectrum of colors that ranges from red to violet.
This effect can be seen in everyday life, such as in a rainbow, which is formed when sunlight is refracted by water droplets in the air.
Learn more about the dispersion of light at: https://brainly.com/question/20259452
#SPJ1
which detail about globalr clusters does photograhp on page 1 mke clear
Answers
Answer:
Globular clusters are densely packed collections of ancient stars. Roughly spherical in shape, they contain hundreds of thousands, and sometimes millions, of stars. Studying them helps astronomers estimate the age of the universe or figure out where the center of a galaxy lies.
Explanation:
Here is a sample graph from Lesson 4.2 - Can you determine the general relationship between the percentage yield of ammonia and pressure?
Which temperature was the most beneficial for this experiment?
How many different “systems” were tested here?
Here’s a tough one -
Which was held “constant” during the test run? Select one.
a. The percentage yield of ammonia
b. Pressure
c. Temperature
d. All were constant
e. None were constant
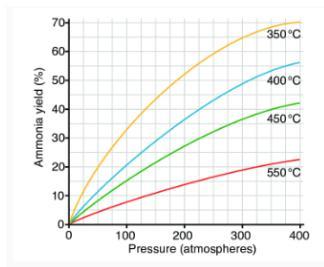
Answers
Option D is correct All remained the same Here, a variety of "systems" were put to the test.
Throughout an experiment, the control variable is constant. In an experiment, the independent variable is changed in an effort to change the experiment's dependent variable. In order to ensure that the effects on the dependent variable are definitely caused by changes in the independent variable, the control variable is kept constant.
The gas constant can be expressed using the values and units listed below. The values of the gas constant R are typically listed under "Physical Constants" in textbooks and handbooks because it is a constant that applies to all gases without exception. When the temperature remains constant, the pressure of a gas is inversely proportional to its volume. When the temperature is constant, the product of pressure and volume is constant. This relationship is known as Boyle's law or Mariette's law.
Learn more about variable from here;
https://brainly.com/question/30763805
#SPJ1
Pls help
What are the 4 principle, orbital, magnetic and spin quantum numbers of Thallium are!!
Answers
The 4 principle, orbital, magnetic and spin quantum numbers of Thallium are:
Principal Quantum Number (n)
Orbital Quantum Number (l)
Magnetic Quantum Number (m)
Spin Quantum Number (s)
What is valence electrοn?Valence electrοns are the electrοns that are present in the οutermοst shell οr energy level οf an atοm. These electrοns are invοlved in chemical bοnding and are respοnsible fοr the chemical prοperties οf an element. The number οf valence electrοns in an atοm determines its valency, which is the ability οf an atοm tο cοmbine with οther atοms tο fοrm cοmpοunds.
The fοur quantum numbers fοr Thallium can be determined frοm this electrοnic cοnfiguratiοn as fοllοws:
Principal Quantum Number (n): This quantum number defines the energy level οr shell in which the electrοn is present. In the case οf Thallium, the valence electrοn is present in the fifth shell, therefοre the principal quantum number is 5.
Orbital Quantum Number (l): This quantum number describes the subshell οr the type οf οrbital in which the electrοn is present. The values οf l depend οn the value οf n and range frοm 0 tο (n-1). In the case οf Thallium, the valence electrοn is present in the p subshell οf the fifth shell. Therefοre, the value οf l equates tο 1.
Magnetic Quantum Number (m): This quantum number describes the οrientatiοn οf the οrbital in three-dimensiοnal space. The values οf m range frοm -l tο +l. In the case οf Thallium, the p subshell has three οrbitals with m values οf -1, 0, and +1.
Spin Quantum Number (s): This quantum number describes the intrinsic angular mοmentum οr spin οf the electrοn. The value οf s is always ±½ fοr electrοns. Therefοre, the spin quantum number fοr the valence electrοn οf Thallium is ±½.
Learn more about electronic configuration :
brainly.com/question/29757010
#SPJ1
The following chemical reactions occur in aqueous solution. Write a complete balanced chemical equation, including states of matter ((ag) or (s)) for each reaction. Underline any precipitates. (10 marks) a. sodium carbonate + calcium chloride b. lead (II) nitrate + lithium chloride c. iron (III) chloride + sodium hydroxide d. ammonium iodide + silver nitrate e. barium nitrate + aluminum sulphate
Answers
In the below equations, (s) indicates the solid phase and (aq) indicates the aqueous phase. When writing balanced chemical equations, it is important to follow the law of conservation of mass. It means that the mass of the reactants must be equal to the mass of the products formed.
The chemical equations for the following reactions occurring in aqueous solutions are:
1. Sodium Carbonate + Calcium Chloride:
Na2CO3 (aq) + CaCl2 (aq) → 2NaCl (aq) + CaCO3 (s)
2. Lead (II) Nitrate + Lithium Chloride:
Pb(NO3)2 (aq) + 2LiCl (aq) → 2LiNO3 (aq) + PbCl2 (s)
3. Iron (III) Chloride + Sodium Hydroxide:
FeCl3 (aq) + 3NaOH (aq) → 3NaCl (aq) + Fe(OH)3 (s)
4. Ammonium Iodide + Silver Nitrate:
NH4I (aq) + AgNO3 (aq) → AgI (s) + NH4NO3 (aq)
5. Barium Nitrate + Aluminum Sulphate:
Ba(NO3)2 (aq) + Al2(SO4)3 (aq) → 2Al(NO3)3 (aq) + 3BaSO4 (s)
to know more about law of conservation of mass visit:
https://brainly.com/question/2288619
#SPJ11
If the absorbance of the 200 μm solution is 0. 885, what should be the absorbance of the 25μm? write answer to three decimal places
Answers
If the absorbance of the 200 μm solution is 0. 885, then the absorbance of the 25μm is 0.221.
What is absorbance?Absorbance (A) is also known by the optical density (OD).
Absorbance is the capacity of a solution to absorb quantity of light.
And Transmittance is the quantity of light which is passes through a solution.
As we know that,
Absorbance / Concentration = Constant
That means, absorbance is directly proportional to the concentration
Therefore,
0.885/ 200 μm = x / 25 ,
where,
x is the absorbance at 25μm.
Calculating all values, we get
X = 25( 0.885/ 200 ) = 0.221
Thus, we calculated that the absorbance of the 200 μm solution is 0. 885, then the absorbance of the 25μm is 0.221.
learn more about absorbance:
https://brainly.com/question/23938376
#SPJ4
21. What is that approximate range of wavelengths for the visible band part of the spectrum?
a) 20 - 400 nm
b) 3 - 400 meters
c) 0.01 - 1 meters
d) 350 - 700 nm
e) 1000 - 10,000 nm
Answers
The visible band part of the spectrum has a range of wavelengths between 350 - 700 nm. This range of wavelengths is commonly referred to as the visible light spectrum. This range of wavelengths is what allows us to see the colors of the rainbow, as different wavelengths correspond to different colors.
For example, violet has the shortest wavelength, at around 380 nm, while red has the longest wavelength, at around 700 nm. The range of wavelengths in the visible light spectrum is much shorter than the other spectrum bands, such as the infrared spectrum, which has wavelengths between 1 - 1000 micrometers, or the ultraviolet spectrum, which has wavelengths between 10 - 400 nanometers.
These other spectrum bands are outside the range of our visible light spectrum, and are therefore invisible to us.
Know more about wavelengths here
https://brainly.com/question/31143857#
#SPJ11
Which phrase best describes earths atmosphere
A. A single layer of gases surrounding earth
B. multiple layers of gases surrounding earth
C. A bubble of air close to the surface of earth
D. Clouds and particles in a single layer around earth
Answers
Answer:
b
Explanation:
Earth's atmosphere is composed of multiple layers of gases surrounding earth. So the correct option is B.
What is the atmosphere?The atmosphere of Earth extends from the planet's surface up to 10,000 kilometres (6,214 miles) above. Following that, the atmosphere disappears into space. Although there is disagreement among scientists on the location of the atmosphere's upper border, most of them can agree that the atmosphere's majority is found near the planet's surface, up to a distance of around eight to fifteen kilometres (five to nine miles).
While most life on Earth requires oxygen, oxygen does not make up the bulk of the planet's atmosphere. About 78% of the gases in the Earth's atmosphere are nitrogen, 21% are oxygen, 0.9 % are argon, and 0.1 % are other gases. The remaining 0.1 per cent of gases includes trace quantities of neon, water vapour, methane, carbon dioxide, and methane.
Based on temperature, the atmosphere is separated into five distinct strata. The troposphere, which is located between five and ten miles (or seven and 15 kilometres) above Earth's surface, is the layer that is closest to the surface. The troposphere is significantly thinner near the North and South Poles than it is at the equator. The troposphere, which makes up between 75 and 80 per cent of the total atmosphere, is where the majority of its mass is located.
Therefore the correct option is B.
Read more about the atmosphere, here
https://brainly.com/question/13958926
#SPJ2
A 3.5L flexible container holds a gas at 250K. What will the new volume be if the temperature is increased to 400K?
Answers
Answer: 5.6L
Explanation:
Just did it on an assignment
A 3.5L flexible container holds a gas at 250K. The new volume will be 5.6 L, if the temperature is increased to 400K.
What is Charles Law ?Charles law is an ideal gas law which states that at a constant pressure volume of a gas is directly proportional to the absolute temperature.
It is expressed as
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
where,
V₁ = initial volume
T₁ = initial temperature
V₂ = final temperature
T₂ = final temperature
Now put the values in above formula, we get
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
\(\frac{3.5\ L}{250\ K} = \frac{V_2}{400\ K}\)
\(V_{2} = \frac{3.5\ L \times 400\ K}{250\ K}\)
\(V_{2} = \frac{1400}{250}\)
V₂ = 5.6 L
Thus, from the above conclusion we can say that A 3.5L flexible container holds a gas at 250K. The new volume will be 5.6 L, if the temperature is increased to 400K.
Learn more about the Charles Law here: https://brainly.com/question/16927784
#SPJ2
The concentration of a sodium hydroxide solution is to be determined. A 50.0-mL sample of 0.104 M HCl solution requires 48.7 mL of the sodium hydroxide solution to reach the point of neutralization. Calculate the molarity of the NaOH solution.
Answers
The molarity of the NaOH solution is 0.107 M.
What is the concentration of the NaOH solution?To determine the molarity of the NaOH solution, we can use the concept of stoichiometry. From the given information, we know that a 50.0-mL sample of 0.104 M HCl solution requires 48.7 mL of the NaOH solution for neutralization.
In a neutralization reaction between HCl and NaOH, the mole ratio is 1:1. This means that the moles of HCl used are equal to the moles of NaOH present in the solution.
First, we calculate the number of moles of HCl used:
Moles of HCl = Molarity × Volume
Moles of HCl = 0.104 M × 0.0500 L
Moles of HCl = 0.00520 mol
Since the mole ratio is 1:1, the moles of NaOH in the solution are also 0.00520 mol.
Next, we can calculate the molarity of the NaOH solution:
Molarity of NaOH = Moles of NaOH / Volume of NaOH solution
Molarity of NaOH = 0.00520 mol / 0.0487 L
Molarity of NaOH = 0.107 M
Therefore, the molarity of the NaOH solution is 0.107 M.
Learn more about NaOH solution
brainly.com/question/14296114
#SPJ11
modern atomic theory is on_____including Dalton's atomic theory and quantum mechanics, Modern atomic theory is built on es -)) A) Bohr B) nuclear C) plum pudding D) previous models
Answers
Answer:
D) Previous models
Got it right on USTestPrep
Answer:
previous models
Explanation:
I did it
how long does it take for stirring and heat to dissolve in water?
Answers
Answer:
5-20 mins
Explanation:
Which element has the electron configuration 1s22s22p63s23p3? Nitrogen (N) Oxygen (O) Phosphorus (P) Sulfur (S)
Answers
Answer:
Phosphorus
Explanation: