from a qualitative, valence-bond perspective, what is the hybridization of carbon-1 (c1) in 1-butyne?spsp^2sp^3
Answers
The hybridization of Carbon-1 (C1) in 1-butyne from a qualitative, valence-bond perspective is sp.
A theory of chemical bonding in which an orbital of one atom interacts with an orbital of another atom to produce a chemical bond is known as valence bond theory. In contrast to molecular orbital theory, it explains chemical bonds by connecting a molecule's bond energies to its constituent atomic orbitals.
The mixing of atomic orbitals into new hybrid orbitals in the formation of a chemical bond is known as hybridization. The hybrid orbitals are more effective than the original atomic orbitals because they enable the orbitals to point in a direction that is more favorable for chemical bonding.
The difference between sp, sp2, and sp3 hybridization is as follows:
When one s and one p orbital of the same atom mix, sp hybridization occurs. The sp orbitals have a linear arrangement of atoms and a bond angle of 180 degrees.The mixing of one s orbital and two p orbitals results in sp2 hybridization.
The sp2 orbitals have a trigonal planar arrangement of atoms and a bond angle of 120 degrees. The orbitals in sp2 hybridization contain one unpaired electron and form sigma bonds by overlapping end-to-end.One s orbital and three p orbitals mix to create sp3 hybridization.
The sp3 orbitals have a tetrahedral arrangement of atoms and a bond angle of 109.5 degrees. The orbitals in sp3 hybridization contain two unpaired electrons and form sigma bonds and lone pairs by overlapping end-to-end.
Learn more about sp2 orbitals here:
https://brainly.com/question/29607102
#SPJ11
Related Questions
In the laboratory, concentrated hydrogen chloric acid reacted with aluminum. Hydrogen gas was collected over water at 25 degrees Celsius and had a volume of 355 cm33 at a total pressure of 750 mm Hg. The vapor pressure of water at 25 degrees Celsius is 24 mm Hg. Find the partial pressure of hydrogen gas.
Answers
Answer:
i dont no this one plz the question is hard
Which mixture in each set has the lowest pH? Justify your answer. a. 1.0 M MgSO4 or 1.0 M Li C0; b. 1.5 M NH4Cl or 1.5 M NaCl c. 1.0 M NaCl or 1.0 M NaF d. 1.0 M Mg(NO3)2 or 1.0 M KCI e. 1.0 M Ni(NO3)3 or 1.0 M Ca(NO3)2 f. CO2 and water or 02 and water
Answers
The pH is lowest at 1.0 M LiC0. c. The pH is lowest in 1.5 M NH4Cl. The pH is lowest at c. 1.0 M NaF. c. The pH is lowest at 1.0 M Mg(NO3)2. d. The pH is lowest at 1.0 M Ca(NO3)2. f. The pH of CO2 and water is lower than that of O2 and water.
The term "lowest pH" describes the solution's or mixture's most acidic pH value. On a scale from 0 to 14, the pH scale determines the acidity or basicity of a solution, with 7 being neutral, values lower than 7 being acidic, and values higher than 7 being basic. As hydrogen ions (H+) are more abundant than hydroxide ions (OH-) in a solution with the lowest pH, it is said to be extremely acidic. The kind and quantity of a solution's constituents, such as salts or acids, can influence its pH, which can have a significant impact on a variety of chemical and biological processes.
Learn more about The pH here:
brainly.com/question/802202
#SPJ4
a cylinder container can hold 2.45 L of water. its radius is 4.00 cm. What is the volume of it in cubic centimeters.
Answers
Hope this help.
How many molecules are in 35.5 grams of carbon dioxide?
Answers
Answer:
you have to use the avogadro's constant of 6.023×10^23 to calculate the number of molecules of carbon dioxide.and you also have to use the molecular mass which is 44.
35.5/44×6.023×10^23
=4.85×10^23
I hope this helps and sorry if it's wrong
Convert 1000.0 mm/s to km/hr. (1 km= 1000 m, 1 m = 1000 mm, 1 hr = 3600 S )
Answers
Answer:
Explanation:
1 km =1000 m
1km=1000000 mm then
1mm=1/1000000=1*10^-6
1 hour =3600 sec
1 sec=1/3600=2.8*10^-4 hr
now
1000.0 km/hr=1000.0*1*10^-6/2.8*10^-4=3.571 mm/s
how many elements have been discovered so far by scientists
Answers
Answer:
118 elements have been discovered so far
Explanation:
in the krebs citric acid cycle, how much of the original carbonyl carbon from acetyl-coa will remain in oxaloacetate after two full cycles?
Answers
None, it will all be lost as Carbon dioxide.
Krebs or citric acid cycle is also known as The tricarboxylic acid (TCA) cycle.
The main source of energy for cells and an important part of aerobic respiration. Aerobic respiration is the respiration occurs in the presence of oxygen.
This cycle harnesses the available chemical energy of the acetyl coenzyme A (acetyl CoA) into the reducing power of nicotinamide adenine dinucleotide (NADH).
TCA cycle metabolizes acetate that is derived from carbohydrates, proteins, and fats to form adenosine triphosphate (ATP) which is the body's energy currency.
To know more about TCA cycle:
brainly.com/question/15561070
#SPJ4
the two electrodes of an electrolytic cell are placed in a sample of molten zinc iodide. after a time, reddish-brown i2(s) begins to form at one electrode while gray zn(s) deposits on the other.
Answers
In the given electrolytic cell setup having molten zinc iodide as the electrolyte, reduction of zinc ions to gray coloured zinc occurs at the cathode, whereas iodide ions get oxidized to reddish-brown iodine at the anode.
An electrolytic cell is a type of electrochemical cell that uses an external source of electrical energy to power a chemical process that would not occur otherwise. This contrasts with a galvanic cell, which serves as a power source and the cornerstone of a battery.
Any apparatus in which electrical energy is changed into chemical energy or vice versa is an electrolytic cell. Such a cell normally consists of two electrodes, which can be metallic or electronic conductors, kept apart from one another and in contact with an electrolyte, which is commonly an ionic substance that has been dissolved or fused.
In the given setup, the molten zinc iodide is the electrolyte, and the half reactions occurring at the two electrodes of the electrolytic cell are:
Half reaction at the cathode:
\(Zn^{2+} (l) + 2e^- \rightarrow Zn (s)\)
Half reaction at the anode:
\(2I^- (l) \rightarrow I_2 (s) + 2e^-\)
Learn more about Electrolytic cells here:
https://brainly.com/question/13361525
#SPJ4
For each of the following solutions, state whether the solution is saturated, unsaturated or supersaturated in the following conditions:
A) at 30o C, 70 g of KNO3 is dissolved in 100 g of water ________________________________
B) at 20 oC 0 g of KClO3 is dissolved in 100 g water________________________________
C) at 100 oC , 30 g of NaNO3 is dissolved in 100 g of water_____________________________
D) 50 g of NH4Cl at 70o C is dissolved in 100 g water. ________________________________
Answers
Answer:
C.at 100•C.30 g of NaNO3 is dissolved in 100 g of water
what factors determine the amount of radiant energy we will receive on earth?
Answers
Describe the results of the experiment that proved atoms contain positive,negative, and neutral particles
Answers
Explanation:
J.J. Thomson's experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. ... Rutherford's gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus.
there u go :>
The experiment that proved atoms positive, negative, and neutral particles is the J.J. Thomson's experiments with cathode ray tubes, which showed that all atoms contain negatively charged s electrons, on the other hand Rutherford's gold foil experiment showed the atom is present in mostly empty space with a tiny, dense, positively-charged nucleus.
what is J.J. Thomson's experiments ?J J Thomson's experiment was used to determine the existence of corpuscles or electrons which says the cathode rays are charges of negative electricity which is carried by particles of matter.
He studied about electric discharge with high vacuum cathode ray tube and there were many other scientists who also have done the same experiment on the similar field.
Cathode ray is made up of of negatively-charged particles and these particles must exist as part of the atom and the mass of the particle is only ∼ 20001 start fraction, 1, divided by, 2000, end fraction the mass of a hydrogen atom.
Learn more about J.J. Thomson's experiments, here:
https://brainly.com/question/14358777
#SPJ6
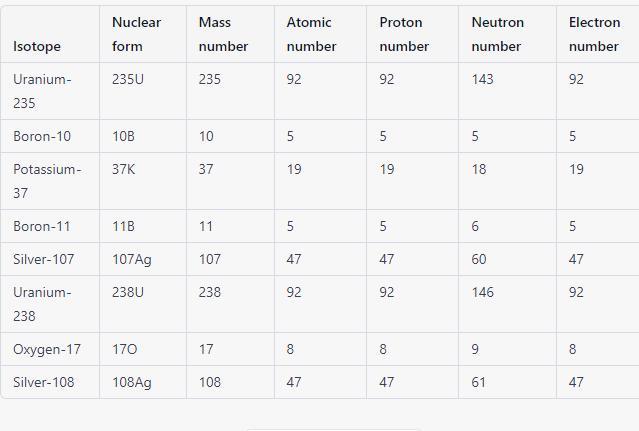
i need this quickly.

Answers
The completed table of the isotopes of the given elements is found in the attachment.
What are isotopes?Isotopes are variations of chemical elements that have a varying number of neutrons but the same number of protons and electrons. In other words, isotopes are different forms of the same element that have different amounts of nucleons (the sum of protons and neutrons) because of variations in the total number of neutrons in each of their individual nuclei.
For instance, the carbon atoms carbon-14, carbon-13, and carbon-12 all exist. A sum of 8 neutrons are present in carbon-14, 7 neutrons are present in carbon-13, and 6 neutrons are present in carbon-12.
Learn more about isotopes at: https://brainly.com/question/14220416
#SPJ1
What kind of reaction does this make?2 C₅H₅ + Fe ⟶ Fe(C₅H₅)₂A. Synthesis (S)B. Decompostion (D)C. Single Displacement (SD)D. Double Displacement (DD)E. Combustion (C)
Answers
The answer is option
The reaction:
\(2C_{5_{}}H_5+Fe\rightarrow Fe(C_5H_5)_2\)is a Synthesis reaction, because from 2 different substances it is produced
which of these pairs of elements is least likely to combine to form an ionic bond?a. carbon and oxygen
b. sodium and fluorine
c. sulfur and hydrogen
d. phosphorus and oxygen
Answers
Answer:(part a) Lithium and Chlorine, (part c) Potassium and Oxygen are likely to form an ionic compound.
Explanation:
brainliest please
Please help me with dis

Answers
Answer:
c
Explanation:
when phenol is treated with the exces s bromine water it gives
Answers
Answer:
bromophenol
Explanation:
when phenol is treated with excess bromine water, it gives bromophenol. This reaction is an example of electrophilic substitution in which the phenol molecule acts as an electron-rich substrate and the bromine in bromine water acts as an electrophile, leading to the substitution of a hydrogen atom in phenol with a bromine atom.
Answer:
it gives 2,4,6-Tribromophenol.
How many moles are present in 1.39 grams of carbon?
Answers
C
R. F. M (Relative Formula Mass) of C=12
12g of C=1 mole
1.39g of C=(1*1.39)/12
=0.1158 moles
a 50 kg laboratory worker is exposed to 20 mj of neutron radiation with an rbe of 10. What is the dose in mSv?
Answers
The given mass of the laboratory worker is 50 kg and the radiation that they were exposed to is 20 mj of neutron radiation with an rbe of 10. We have to find the dose in mSv.
The dose equivalent can be calculated using the formula, Given, Mass of the worker, m = 50 kg Energy absorbed, E = 20 MJRBE (Relative Biological Effectiveness) = 10 We have,1 Sv = 1 Gy x Q, where Q is a quality factor. As per the question, the RBE value is 10 (for neutron radiation).
Now,1 Sv = 1 Gy x Q = 1 x 10 = 10 Gy From the formula, Dose equivalent = Energy absorbed / mass of the worker x RBEWe know, 1 Gy = 1 J/kg∴ Energy absorbed = 20 x 10^6 J Mass of the worker = 50 kgRBE = 10Dose equivalent = Energy absorbed / mass of the worker x RBE= (20 x 10^6) / (50 x 10^3) x 10= 40 mSvTherefore, the dose in mSv is 40.
To know more about radiation visit :
https://brainly.com/question/31106159
#SPJ11
Write about the various sequential steps of scientific research.
Answers
Create a hypothesis
Test it out
Analyze and collect the data from the experiment you do to test your theory’s correct.
Come to a conclusion on if you were right or wrong
There are seven sequential steps of scientific research.
What is Scientific Research?The scientific method is a process used when conducting experiments and exploring observations. Some areas of science rely more heavily on this method to answer questions, as they are more easily tested than other areas.
This method aims to discover the relationships between cause and effect in various situations and applications. The 7 steps of scientific research are -
Ask a questionPerform researchEstablish hypothesisTesting hypothesis by conducting an experimentMake an observationAnalyze the results and draw a conclusion.Therefore, there are seven sequential steps of scientific research.
Learn more about Scientific research, here:
https://brainly.com/question/30547498
#SPJ2
what is the solute when stirring salt in water until the salt disappears?
Answers
Answer:
The solute is the substance being dissolved.
The solvent is the substance dissolving the solute.
Therefore, the salt is the solute and the water is the solvent.
Explanation:
The salt is the solute.
A weather map of Chicago with a high pressure system and warm front. Based on the weather map, what might the upcoming weather be like in Chicago? Warm, dry, clear skies Warm, humid, possible thunderstorms Cold, dry, clear skies Cool, humid, possible thunderstorms.
Answers
A weather map of Chicago with a high pressure system and warm front, so the upcoming weather be Warm, dry, clear skies in Chicago.
What is high pressure of air?High pressure of air tries to compress the gas from upper atmosphere to lower atmosphere.
When any gas has high pressure then it sinks towards the land from the upper atmosphere, and at the upper part air gets cool and form water vapor. When air comes towards the land then, it becomes warm and dry as it doesn't participate in the formation of precipitate. Due to which we are able to see clear sky at the dry days.
Hence, option (1) is correct i.e. Warm, dry, clear skies are the upcoming weather.
To know more about pressure of air, visit the below link:
https://brainly.com/question/441281
Answer:
A. Warm, dry, clear skies
( Hope this helps ) <3 ( Give person above brainliest )

glyphosate is a common compound found in many herbicides. why was it thought to have no effect on human health before recent scientific studies?
Answers
Glyphosate was initially thought to have no effect on human health because it primarily targets enzymes found only in plants and bacteria, not humans.
Additionally, the compound was believed to have a low toxicity level and was considered to be safe when used according to the labeled instructions.
However, recent scientific studies have suggested potential health risks associated with glyphosate exposure, including links to cancer and other health issues.
These studies have prompted further investigation and controversy surrounding the safety of glyphosate in herbicides.
To know more about Glyphosate, refer here:
https://brainly.com/question/30628930#
#SPJ11
Which is the best example of the use of imagery in a sentence?
Answers
Answer:
Explanation: As the good witch Azura said when she was in the bog of immediate regret I challenge you to a witches duel.
im sorry if that was bad i got nothing lol
also took it from The Owl House
HELP ASAP
how is human activity creating water pollution and depletion, and how can these effects be addressed while using water sustainably
Answers
The human activity creating water pollution and depletion are uses of fertilizers, detergents and chloro flouro carbon many other things.
What is water pollution?Water pollution is defined as the contamination of water bodies, usually as a result of human activity, in such a way that their lawful uses are harmed.
The activities of human that creating water pollution are:
Uses of fertilizersHarmful chemical in agricultureDetergents for washing clothesWastes from industriesAnd depletion in the ozone layer are due to halogen source gases, which contain chlorine and bromine atoms, are produced by human activities. These emissions into the atmosphere eventually contribute to ozone depletion in the stratosphere.
Hence human activities are uses of fertilizers, detergents and many other things.
To know more about water pollution, visit the below link:
https://brainly.com/question/2165740
#SPJ1
. (i) how does the number of atoms in a 27.5-gram gold ring compare to the number in a silver ring of the same mass?
Answers
The silver ring of the same mass of 27.5 gm of gold will have 1.9 times more atom.
We need to find moles first,
Moles = mass/ Atomic Mass
Then number of atoms can be calculated from moles,
So , no. of moles of gold ring = 27.5g/196.967
= 0.13 moles.
Since 1 mole contains 6.023 x 10^23 atoms, then 0.13 mole contains (6.023 c 10^23) x 0.13 atoms
= 7.82 c 10^22 atoms.
no. of moles for silver = 27.5/107.87
= 0.25 moles.
This contains (6.023 x 10^23) x 0.27 atoms.
= 1.5 x 10^23 atoms.
So 27.5g of silver contains 1.67 x 10^23/9.17 x 10^22 more atoms than 27.5g of gold.
So 27.5g silver contains 1.9 x more atoms than 27.5g of gold.
To know more about atoms, click here,
brainly.com/question/6258301
#SPJ4
in a reaction 2.5g of sodium sulphate reacted with 4.5g of barium chloride. the products are 3.5g of barium sulphate and the rest is sodium chloride. find the mass of sodium chloride produce. state the law which justifies this reaction
Answers
The mass of sodium chloride : 3.5 g
Further explanationGiven
Reaction :
2.5g of sodium sulphate + 4.5g of barium chloride ⇒ 3.5g of barium sulphate + sodium chloride
Required
The mass of sodium chloride
Solution
Conservation of mass applies to a closed system, where the masses before and after the reaction are the same
From the reaction :
mass of reactants = 2.5 g + 4.5 g = 7.0 g
mass of products = 3.5 g + mass of sodium chloride
mass of reactants = mass of products
7.0 g = 3.5 g + mass of sodium chloride
mass of sodium chloride = 7 g - 3.5 g =3.5 g
You are riding a bicycle. if you apply a forward force of 125 n, and you and
the bicycle have a combined mass of 82 kg, what will be the forward
acceleration of the bicycle? (assume there is no friction.)
o a. 1.52 m/s2
b. 1.67 m/s2
c. 3.37 m/s2
d. 0.66 m/s2
Answers
The forward acceleration of the bicycle is 1.52 m/s². Option A is correct.
Forward acceleration refers to the rate at which an object's velocity increases in the forward direction. It is measured in units of meters per second squared (m/s²).
To find the forward acceleration of the bicycle, we need to use Newton's second law of motion, which states that force is equal to mass times acceleration (F=ma).
Given:
Force (F) = 125 N
Combined mass (m) = 82 kg
Using the formula:
a = F/m
Substituting the values, we get:
a = 125 N / 82 kg
a = 1.52 m/s²
Hence, A. 1.52 m/s² is the correct option.
To know more about forward acceleration here
https://brainly.com/question/7694093
#SPJ4
Part 1: Name two elements that have the same properties as magnesium (Mg). (4 points)
Part 2: Determine the number of protons, electrons, and neutrons present in an atom of potassium (K). Explain how you determined your answer using complete sentences. (6 points)

Answers
Two elements having same properties as magnesium are calcium and strontium. The number of protons and neutrons in potassium is 19 and number of neutrons is 20.
What is periodic groups?Groups in periodic table are vertical columns with elements of similar physical and chemical properties. All elements are classified into different groups based in the number of valence shell electrons.
Elements of same group have same number of valence electrons. The element magnesium Mg have 12 electrons with 2 valence electrons. Its group members are shown under Mg in the column and they are calcium, strontium and rubidium.
Number of electrons in an tom is equal to the number of protons and this is called the atomic number. The atomic number o potassium is 19 and it have 19 electrons and protons.
Number of electrons = mass number-number of protons
= 39 -19 =20.
Hence, potassium (K) have 19 electrons and protons and 20 neutrons.
To find more about potassium, refer the link below:
https://brainly.com/question/13321031
#SPJ1
A 25g sample of calcium oxide is heated with excess HCl to produce water and 37. 5g of calcium
chloride. What is the percentage yield of the reaction?
Answers
The percentage yield of the reaction is 76.1, when the actual yield and theoretical yield is 37.5 g and 49.28 g respectively.
First of all we would write a balanced chemical equation for the reaction between calcium oxide (CaO) and hydrochloric acid (HCl):
CaO + 2HCl → CaCl₂ + H₂O
We can now see that 1 mole of CaO reacts with 2 moles of HCl to produce 1 mole of CaCl₂ and 1 mole of H₂O.
Calculating the theoretical yield of CaCl₂:
Calculate the number of moles of CaO:m(CaO) = 25 g / 56.08 g/mol = 0.445 mol
Use the mole ratio between CaO and CaCl₂ to calculate the number of moles of CaCl₂:n(CaCl₂) = n(CaO) = 0.445 mol
Calculate the mass of CaCl₂:m(CaCl2) = n x M
= 0.445 mol x 110.98 g/mol = 49.28 g
Therefore, the theoretical yield of CaCl₂ is 49.28 g.
To find the percentage yield of the reaction:
% yield = (actual yield / theoretical yield) x 100
The actual yield of CaCl₂ is given as 37.5 g.
% yield = (37.5 g / 49.28 g) x 100
= 76.1%
Therefore, the percentage yield of the reaction is 76.1.
To know more about percent yield, refer:
https://brainly.com/question/19734683
#SPJ4
What are the two groups the animal kingdom is divided into?
Answers
Answer:
Taking the animal kingdom as an example, we can see that it is split into two clear groups: Invertebrates - animals without a backbone. Vertebrates - animals with a backbone.
Explanation: