Given 10.0 g of each of the following elements (N2, O2, C, S) choose the correct answer from the following choices. Highest number of atoms: Choose... + Greatest molar mass: Choose... Greater number of molecules: Ny or O, Choose.... Calculate the number of moles of Natoms in 1.00 x1022 Ny molecules.
Answers
The number of moles of N atoms in 1.00 x1022 Ny molecules is 1.66 ₓ 10⁻² mol.
What do you mean by mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.
1 mole is equal to 6.023 × 10 ²³ molecules.
10 gm of Nitrogen gas having number of mol = mass/ molar mass
n =10 / 28
n = 0.3571 mol
number of nitrogen molecule is 0.3571 × 6.0221023 molecules
= 2.151023 molecules
number of nitrogen atoms = 20.3571 × 6.0221023 atoms
= 4.301023 atoms
b. 10 gm of oxygen gas having no of mol = mass / molar mass
n = 10/32
n= 0.3125 mol
number of oxygen atoms = 0.3125 × 6.0221023 molecules
= 1.881023 molecules
number of oxygen atoms = 20.3125 × 6.0221023 atoms
= 3.761023 atoms
c. 10 gm of carbon having no of mol = mass / molar mass
n = 10 / 12
n= 0.83 mol
number of Carbon atoms = 0.83 × 6.0221023 atoms
= 4.9991023 atoms
d. 10 gm of sulfur having no of mol = mass / molar mass
n = 10 / 32
n= 0.3125 mol
number of nitrogen atoms = 0.3125 × 6.0221023 atoms
= 1.881023 atoms
So, the highest number of atom is 10 gm Carbon.
and greatest molar mass has sulfur and oxygen if sulfur is taken as octaatomic as sulfur has greatest molar mass.
here nitrogen has the greatest number of molecule.
Therefore, 6.022× 10²³ molecules of N2 gas contain 1 mol
so, 1 × 10²² molecule of N2 gas having
= 0.166 ₓ 10-1
= 1.66 ₓ 10⁻² mol.
Thus, the number of moles of N atoms in 1.00 x1022 Ny molecules is 1.66 ₓ 10⁻² mol.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Related Questions
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
What mass of carbon dioxide is produced from the complete combustion of 6.60×10−3 g of methane?
Answers
0.01815g CO2
Explanation:
Combustion of Methane:
CH4+2O2—>CO2+2H2O
Consider 6.60x10-3g or 0.0066 of CO
Now, divide the mass of CH4 which is 16g and CO2 which is 44g
And multiply by 0.0066 of CO
Guys I need help with this ASAP! An atom of Iron has a mass of 56 atomic mass units. What is the number of protons and the number of neutrons in the nucleus?
Answers
Answer:
protons = 26; neutrons = 30
Explanation:
protons = atomic number; neutrons = atomic mass – # of protons.
Explain an example of radiation when you feel heat without directly touching it.
Answers
Explanation:
In fact, a good way to remember radiation is that it is how you can feel heat without touching it. Heat passes through the empty space until it reaches your hand. That's radiation! A fire is another example of radiation.
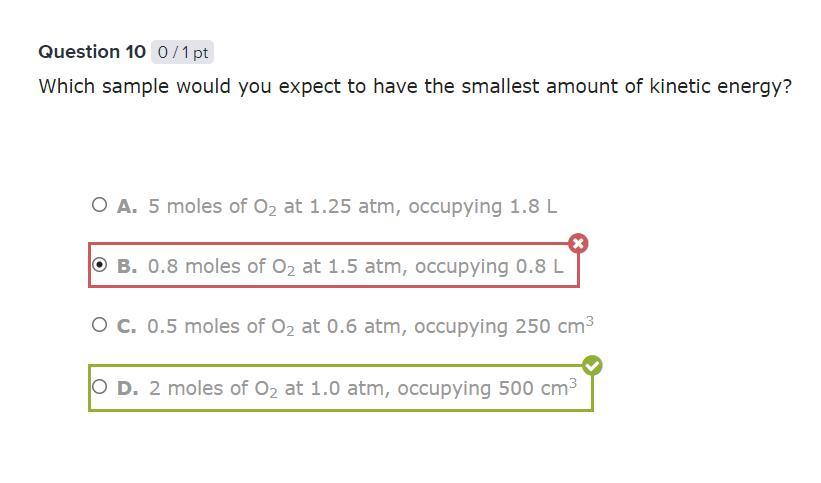
Which sample would you expect to have the smallest amount of kinetic energy?
THIS IS THE ANSWER. NOT A QUESTION
Answers
Answer:
ok so what do i do now that you posted the answer?
Answer: D
Explanation:
definitely didn't copy you
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
Another sample of eggshell reacts completely with 4.0 mL of an HCl(aq) solution of unknown concentration. If the reaction produced 0.095 atm of gas, the concentration of the HCl(aq) solution was at least (A) 0.0020 M (B) 0.050 M (C) 0.50 M (D) 1.0M
Answers
The concentration of the HCl (aq) solution was at least 1.0 M.
What is concentration?The abundance of a constituent divided by the sum of the mixture's volumes is the definition of concentration in chemistry. There are several different categories of mathematical description: mass concentration, molar concentration, number concentration, and volume concentration
P = 0.095atm(corresponds to 0.20g of CaCO3
so,moles of CaCO3 =0.20g/100gmol⁻¹=0.002moles
moles of HCl =(molesₓCaCO3ₓ2)=0.002ₓ2
M=0.004/4ₓ100
Concentration of HCl=1M
For more information about concentration please visit:
https://brainly.com/question/10725862
#SPJ4
When extended, a bicycle pump has a volume of 0.952 L at STP. What is its pressure when the pump is compressed to a new volume of 0.225 L and 278 K? (Think Combined)
Answers
We can assume that the gas inside the bicycle pump does not change its number of moles. Furthermore we will assume the gas behaves as an ideal gas so it is described by the following equation:
\(\begin{gathered} PV=nRT \\ n=\frac{PV}{RT} \end{gathered}\)Where,
P is the pressure of the gas
V is the volume of the gas
T is the temperature of the gas
n is the number of moles of the gas
R is a constant =0.08206 atm.L/ol.K
Now, we have two states, state 1 at STP conditions and state 2. Since the number of moles remains constant we can equate the two states.
\(\begin{gathered} \frac{P_1V_1}{RT_1}=n=\frac{P_2V_2}{RT_2} \\ \frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} \end{gathered}\)Now, the conditions for each state are:
State 1.
V1=0.952L
T1=273.15K (STP)
P1=1atm (STP)
State 2.
V2=0.225L
T2=278K
P2=?
We clear V2 and replace the known data:
\(P_{2=\text{ }}\frac{P_1V_1}{T_1}\times\frac{T_2}{V_2}\)\(\begin{gathered} P_{2=\text{ }}\frac{1atm\times0.952L}{273.15K}\times\frac{278K}{0.225L} \\ P_{2=\text{ }}4.31atm \end{gathered}\)Answer: The new pressure will be 4.31atm
2 Select all the correct answers. A worker is holding a filled gas cylinder still. Which two sentences are true about the energy of the filled gas cylinder? It has no energy because it’s being held still. It has gravitational potential energy because of its height. Its atoms and molecules have thermal energy. It has motion energy because it will fall if let go. Its kinetic energy is being converted to potential energy.
Answers
The sentences that are true about energy in a gas cylinder are:
It has gravitational potential energy because of its height.Its kinetic energy is being converted to potential energyWhat is energy?Energy is the ability or capacity of people or a thing to do work or perform task effectively.
Therefore, The sentences that are true about energy in a gas cylinder are:
It has gravitational potential energy because of its height.Its kinetic energy is being converted to potential energy.Learn more about energy below.
https://brainly.com/question/13881533
#SPJ1
A gas sample has a temperature of 24 ∘C with an unknown volume. The same gas has a volume of 464 mL when the temperature is 81 ∘C, with no change in the pressure or amount of gas.What was the initial volume, in milliliters, of the gas?Express your answer to three significant figures and include the appropriate units.
Answers
Explanation:
We have a sample of gas that has a temperature of 24°C and an unknown volume. The same sample at the same pressure has a volume of 464 mL at 81°C. We have to find the initial volume.
To solve our problem we can apply the formula that shows us the relationship between the volume and temperature of a gas sample at constant pressure.
V₁/T₁ = V₂/T₂
Where V₁ and T₁ are the initial temperature and volume and V₂ and T₂ are the final volume and temperature. We already know some of these values, but we have to convert the temperatures from °C to Kelvins, because we have to work with an absolute temperature scale (to not divide by zero).
V₁ = ?
T₁ = (273.15 + 24) K
T₁ = 297.15 K
V₂ = 464 mL
T₂ = (273.15 + 81) K
T₂ = 354.15 K
Finally we can replace these values in the formula and solve it for V₁ to get the answer to our problem.
V₁/T₁ = V₂/T₂
V₁ = V₂ * T₁/T₂
V₁ = 464 mL * 297.15 K/(354.15 K)
V₁ = 389 mL
Answer: the initial volume was 389 mL.
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
A block of aluminum occupies a volume of 10mL and has a mass of 40 g.
What is its density? (D = M/V)
Answers
Density of a block of aluminum occupies a volume of 10mL and has a mass of 40 g is 4g/ml.
Define density.
A material's density is determined by how closely it is packed. As the mass per unit volume, it has that definition. The relationship between density and volume and mass is inverse.
The density rises if the volume remains constant while the amount of matter increases. Density drops when volume grows without accompanying increases in mass. When a substance is heated, the molecules move faster and slightly farther apart, taking up more space and causing the density to drop. When something is cooled, the molecules slow down and get a little closer together, taking up less space and becoming denser.
D = M/V
Mass, M is 40 g.
Volume, V of 10mL
Density, D will be 40/10 i.e. 4g/ml.
To learn more about density use link below:
https://brainly.com/question/26364788
#SPJ1
For each pair circle the neutral element with the largest atomic radius K or Cs ,Ti or As ,Br or F
Answers
Answer:
Cs
Br
Ti
Explanation:
As we move down the group atomic radii increased with increase of atomic number. The addition of electron in next level cause the atomic radii to increased. The hold of nucleus on valance shell become weaker because of shielding of electrons thus size of atom increased. In this way atomic radii of Cs is larger than K and size of Br is larger than F.
As we move from left to right across the periodic table the number of valance electrons in an atom increase. The atomic size tend to decrease in same period of periodic table because the electrons are added with in the same shell. When the electron are added, at the same time protons are also added in the nucleus. The positive charge is going to increase and this charge is greater in effect than the charge of electrons. This effect lead to the greater nuclear attraction. The electrons are pull towards the nucleus and valance shell get closer to the nucleus. As a result of this greater nuclear attraction atomic radius decreases
Thus size of As is smaller than Ti.
On Earth he weighs 720 newtons. List of weights: 655 N; 1,872 N; 792 N; 36 N; and 661 N. What planets does he visit?
Answers
On Earth he weighs 720 newtons. 36N is the weight. Therefore, the correct option is option D among all the given options.
What is weight?The gravitational force of attraction exerted on an item by the presence of a huge second object, including the Earth or Moon. Weight is a result of the fundamental law of gravitation: whatever two things have the same weight.
They attract each other using a force that really is directly related to the sum of their masses as well as inversely related to the square of something like the distance separating them due to their masses.
F = mass ×4/9g
= 720 ×4/9
=36N
Therefore, the correct option is option D.
To know more about weight, here:
https://brainly.com/question/12156778
#SPJ9
Copper has a heat capacity of 0.386 J/g °C. If 15 g of copper has a temperature of 180 ºC and it is added to a glass of water causing the temperature to decrease to 80ºC. What is the amount of heat released by the metal?
Answers
Heat = mass * specific heat. According to the definition, a material's specific heat capacity is normally calculated by measuring the heat capacity of a sample of the substance.
What is Heat capacity?Heat capacity = mass* specific heat.
= 15 g * ( 100 c)
= 1500 g c.
When a substance, especially a gas, is heated while being allowed to expand (specific heat capacity at constant pressure) as opposed to being heated in a closed vessel that forbids expansion.
Its specific heat capacity may be much larger (specific heat capacity at constant volume).
Therefore, Heat = mass * specific heat. According to the definition, a material's specific heat capacity is normally calculated by measuring the heat capacity of a sample of the substance.
To learn more about Specific heat, refer to the link:
https://brainly.com/question/11297584
#SPJ1
which force holds distant stars and other matter together?
Answers
Answer:
Gravity is the force that holds everything in the universe together.
Hope that helps!
What are three different ways an animal uses energy from its food
Answers
Answer:
Animals obtain energy from the food they consume , using that energy to maintain body temperature and perform other metabolic functions. Glucose, found in the food animals eat ,is broken down during the process of cellular respiration into energy source called ATP.
What are unit conversions used for?
A. Unit conversions associate a number with its units.
B. Unit conversions express an amount in a different unit.
C. Unit conversions are used to write very large numbers.
D. Unit conversions express the fractional size of something.
Answers
Answer:
B. Unit conversions express an amount in a different unit
Do electron pair geometry of Carbonothioyl dibromide please
Answers
Answer:
See below.
Explanation:
To determine the electron pair geometry of Carbonothioyl dibromide, we need to first draw its Lewis structure:
Br Br
\ /
C=S
/
Br Br
Carbonothioyl dibromide has four electron groups around the central sulfur atom: two single bonds with the two bromine atoms, one double bond with the carbon atom, and one lone pair of electrons on the sulfur atom.
Using VSEPR theory, we can predict the electron pair geometry by considering both the bonding and the lone pairs of electrons. In this case, the electron pair geometry of Carbonothioyl dibromide is tetrahedral because it has four electron groups around the central sulfur atom.
However, the molecular geometry of Carbonothioyl dibromide is trigonal pyramidal due to the presence of one lone pair of electrons on the sulfur atom.
someone please help!!
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxygen gas in excess C4H10, how much Carbon Dioxide can be created?
Box 1 = number
Box 2 = units
Box 3 = substance

Answers
Answer:
BOX 2
Explanation:
Which of the following do omnivores eat?
A. only
B. plants and meat
C. meat only
D. they make their own food
Answers
Answer:
(B. Plants and meat)
Explanation:
Which shows an isomer of the molecule below?

Answers
Answer:
D
Explanation:
An isomer is a molecule with the same number of atoms as another compound, but they differ in arrangement of the atoms.
Answer:
D
Explanation:
A P E X
How much does REAL carbon fiber cost ? lets say as big as a piece of paper
Answers
Answer:
Today, the average total production cost of “standard modulus” carbon fiber is in the range of $7-9 per pound.
Methanegasand oxygengas react to form carbon dioxide gas and watervapor. Suppose you have of and of in a reactor. Calculate the largest amount of that could be produced. Round your answer to the nearest .
Answers
Answer:
Kindly check the explanation section.
Explanation:
NB: kindly take note that the question is not complete since we do not have any values for methane gas and water. I tried looking for the complete question but, I was unable to get it.
So, let's make use of the values of 7mols for Oxygen gas and 11 moles for the methane gas.
Therefore, the chemical equation for the reaction is given below:
CH4(g) + 2O2(g) -------------> CO2(g) + 2H2O.
From the equation of reaction we have that 1 mole of Methane, CH4 reacts with 2 moles of Oxygen,O2 to give 2 moles of water, H2O and one mole of Carbondioxide,CO2. So, we have the ratio= 1 : 2 : 1 : 2.
The next thing for us to do is to find the limiting reagent which can be determine by calculating the number of moles In each reactants.
Number of moles of O2 = 7 moles
Number of moles of methane, CH4 = 11/1 = 11 moles.
Thus, the limiting reagent is Oxygen.
The largest amount of CO2 that could be produced is 7 moles of O2 × (1 mole of CO2/ 2 mol O2. = 3.5 moles of CO2.
A sample of pure tin metal is dissolved in nitric acid to produce 15.00 mL of solution containing Sn2+. When this tin solution is titrated, a total of 42.1 mL of 0.145 mol/L KMnO4 is required to reach the equivalence point. a. What is the concentration of the Sn2+ solution?b. Find the concentration of the Sn2+(aq) in mol/L: (give your answer to 3 decimal places)
Answers
Answer:
1.00 M
Explanation:
Sn^2+ reacts with KMNO4 as follows;
5Sn^2+(aq) + 2MnO4^-(aq) + 16H^+(aq) ----> 5Sn^4+(aq) + 2Mn^+(aq) + 8H2O(l)
The number of moles of MnO4^- reacted = 42.1/1000 L × 0.145 mol/L
= 0.0061 moles
If 5 moles of Sn^2+ reacts with 2 moles of MnO4^-
x moles of Sn^2+ reacts with 0.0061 moles of MnO4^-
x= 5 × 0.0061/2
x= 0.015 moles
Since the volume of the Sn^2+ solution is 15.00mL or 0.015 L
number of moles = concentration × volume
Concentration = number of moles/volume
Concentration= 0.015 moles/0.015 L
Concentration = 1 M
What is matter?
O A. Anything that can be seen.
O B. Anything that can be treasured
O C. Anything that takes up space and has mass
B. Anything that has
energy and motion
Answers
Answer:
Anything that takes up space and has mass
C2N2H8 empirical formula
Answers
Answer:
\(\huge\boxed{\sf CNH_4}\)
Explanation:
Empirical formula:The simplest whole number ratio of atoms in a compound is known as empirical formula.Solution:Given compound us,
C₂N₂H₈
Ratio:= 2 : 2 : 8
Divide by 2= 2 ÷ 2 : 2 ÷ 2 : 8 ÷ 2
= 1 : 1 : 4
So, we can write the formula as:
= CNH₄\(\rule[225]{225}{2}\)
How many water molecules are contained in a 0.50L bottle of water? Assume water has a density of 1.00 g/mL.
Answers
18.4 × 10^(24) water molecules are contained in a 0.50L bottle of water.
Given,
Density of water = 1.00 g/mL
Volume of water = 0.50L = 500 ml
We can calculate the mass of water.
As we know that,
Density = mass/ volume
By substituting the values, we get
1 = Mass / 500
Mass = 500 g.
Now, we know
Molar mass of water = 18 g
Mass of water = 500g
We will calculate the moles of water
Mole = given mass / molar mass
= 500/18 = 27.7 moles
As we know that,
1 mole = 6.673 × 10^(23) molecules
So, 27.7 moles contain = 6.673 × 27.7 × 10^(23)
= 18.4 × 10^(24) molecules
Thus, we concluded that 18.4 × 10^(24) water molecules are contained in a 0.50L bottle of water.
learn more about Density:
https://brainly.com/question/952755
#SPJ13
Which of the following would match the electrons in a Rubidium ion? (Select. all that apply)
Xenon
Krypton
Bromine ion
Potassium ion
Iodine ion
Answers
chemistry
Definition in your own words. I will check if you got it from online.
Word: Melting Point
Answers
Answer:
The specification of the query in the discussion is characterized below.
Explanation:
The given term would be the temperature through which the solid, as well as the liquid sequence, could sometimes exist side by side throughout equilibration as well as the atmospheric pressure beyond which the matter keeps changing from a solid-state to the liquid one. The concept shall apply to the liquid phase as well as alternatives. The melting point changes depending on either the pressure, therefore it needs to be clearly stated.