Answers
The heat transferred when 4.5 grams of Carbon reacts with H2O is approximately 42.38 kJ. Therefore, the correct option is 42 kJ absorbed.
Option B.
Given reaction is as follows: C(s) + H2O(g) + 113 kJ → CO(g) + H2(g)To find the amount of heat transferred when 4.5 grams of Carbon reacts with H2O, we have to first find the amount of moles of Carbon present. The molar mass of Carbon is 12 g/mol. Therefore, the amount of moles of Carbon can be calculated as follows:mass of carbon/molar mass of carbon=4.5 g/12 g/mol=0.375 molNow, to find the amount of heat transferred, we use the equation, q = n∆Hwhere q is the heat transferred, n is the amount of moles of Carbon present, and ∆H is the enthalpy change for the given reaction. ∆H is given in the equation as 113 kJ.To find the sign of ∆H, we look at the reactants and products. In the given reaction, Carbon reacts with H2O to form CO and H2. Since Carbon and H2O are reactants and CO and H2 are products, this reaction is an endothermic reaction. Hence, the value of ∆H is positive.∆H = 113 kJ/molNow, substituting the values in the equation, q = n∆Hq = 0.375 mol × 113 kJ/molq = 42.38 kJ (approx)
Option B.
For more questions on heat
https://brainly.com/question/30738335
#SPJ8
Related Questions
hydrochloric acid + __________ —> copper chloride + water
Pls say someone answer science iam confused
Answers
Explanation:
HCl + __ => CuCl2 + H2O
The blank should be the metal, which is copper.
(This reaction is not spontaneous but can happen using electrolysis, etc.)
A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions.
Answers
Answer:
This option is correct
Explanation:
Molecules are expressed in the form of Lewis in order to simplify their expression or chemical study, but this does not mean that they manifest or respect this form in all three dimensions since some are generally unstable, or even in the third dimension, these locations between atoms are respected for the characteristics of their unions.
Absorption is usually measured at a wavelength where.
Answers
Absorption is usually measured at a specific wavelength that corresponds to the maximum absorption of the particular substance being studied. This wavelength is known as the absorption peak or maximum, and it can vary depending on the chemical structure and properties of the substance.
In spectroscopy, the absorption spectrum of a substance is obtained by measuring the amount of light absorbed at different wavelengths. The wavelength at which maximum absorption occurs is an important characteristic used for identification and analysis of various substances. Understanding the absorption properties of substances is crucial for many applications, including drug discovery, environmental monitoring, and materials science. Overall, the measurement of absorption at a specific wavelength is a fundamental concept in chemistry and physics.
Absorption is typically measured at a specific wavelength where the substance being analyzed exhibits the maximum absorption. This is known as the "absorption maximum" or "lambda max" (λmax). By measuring absorption at this particular wavelength, it allows for the most accurate and sensitive determination of the substance's concentration, as the absorbance is directly proportional to its concentration according to the Beer-Lambert Law. This approach also minimizes the potential interference from other substances that may absorb light at different wavelengths.
For more information on Beer-Lambert Law visit:
brainly.com/question/30404288
#SPJ11
Which of these products was made using a renewable resource?
O A. Paper plate
O B. Aluminum can
O C. Copper pan
O D. Plastic bag
Answers
Answer:
paper plate
Explanation:
A renuable source is a source that would replenish in ones lifetime. Trees make paper and grow in the span of a average human life.
what is the molecular formula of benzoyl peroxide (c7h5o2) of the molecular mass is 0.242 kg/mol?(show work please)
Answers
Answer : The molecular formula of benzoyl peroxide is \(C_{14}H_{10}O_4\)
Explanation :
Empirical formula : It is the simplest form of the chemical formula which depicts the whole number of atoms of each element present in the compound.
Molecular formula : it is the chemical formula which depicts the actual number of atoms of each element present in the compound.
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
\(n=\frac{\text{Molecular mass}}{\text{Empirical mass}}\)
As we are given that the molar mass of compound is, 0.242 kg/mol.
Molecular mass = 0.242 kg/mol = 242 g/mol (1 kg = 1000 g)
The empirical mass of \(C_7H_5O_2\) = 7(12) + 5(1) + 2(16) = 121 g/eq
\(n=\frac{\text{Molecular mass}}{\text{Empirical mass}}\)
\(n=\frac{242}{121}\)
\(n=2\)
Molecular formula = \((C_7H_5O_2)_n\) = \((C_7H_5O_2)_2\) = \(C_{14}H_{10}O_4\)
Thus, the molecular formula of benzoyl peroxide is \(C_{14}H_{10}O_4\)
The equation of line v can be written as x+4y=16. Line w, which is parallel to line v, includes the point (−8,4). What is the equation of line w?
Write the equation in slope-intercept form with no spaces. Write the numbers in the equation as simplified proper fractions, improper fractions, or integers.
Answers
the equation of line w is y = -(1/4)x + 2. To solve for the equation of line w, we first need to find the slope of line v. The slope of line v can be found by subtracting the y-coordinates of two points on the line and dividing by the difference of the x-coordinates of those same two points.
In this case, we can use the points (-8, 4) and (0, 0). The slope of line v is then:
m = (4 - 0) / (-8 - 0) = -1/4
We know that line w is parallel to line v, so it will have the same slope. The slope-intercept form of a line is y = mx + b, where m is the slope and b is the y-intercept. We can plug in the slope of line w, which is -1/4, and the point (-8, 4), which is on line w, to solve for b. This gives us:
y = -(1/4)x + b
4 = -(1/4)(-8) + b
4 = 2 + b
b = 4 - 2
b = 2
To know more about slope intercept form, click here:-
https://brainly.com/question/29146348
#SPJ11
Question 14 of 30
Which of the following elements has 1 valence electron?
The Periodic Table
A. Sodium (Na)
B. Chlorine (CI)
C. Calcium (Ca)
D. Helium (He)
SUBMIT
Answers
Answer:
A. Sodium (Na)
Explanation:
The total number of electrons in the outermost principal energy level of an element is called the valence electrons of that element.
Na (11)---→1s2 2s2 2p6 3s1
There 1 electron in the outermost principal energy level of Sodium. So, the number of valence electron of Sodium is 11.

Answer:
Na
Explanation:
I was learning about it recently, that dude's right, it's Sodium
suppose you saw statue made of solid silver medal in the museum US meet at the statues volume of 8 L if you wanted to steal the statue would you be able to lift it by yourself or would you need to bring along a friend to help carry it? The Density of Silver per cm^3 is 10.5. 1 liter is equivalent to 1000cm^3
Answers
The volume of the statue = 8 L
The density of the silver = 10.5 per \(cm^{3}\)
The mass of the statue is x kg
As we know,
\(Density = \frac{Mass}{Volume}\)
Therefore, \(10.5 = \frac{x}{8} \\x = 10.5\) × \(8 = 84 kg.\)
So, the weight of the statue is heavy. You have to took help of your friend to carry the statue.
The density of a substance indicates how dense it is in a particular area. Mass per unit volume is the definition of a material's density. In essence, density is a measurement of how closely stuff is packed.
It is a special physical characteristic of a certain thing. The Greek scientist Archimedes made the discovery of the density principle.
If you are familiar with the formula and the relevant units, calculating density is simple. The letter D can also be used to signify density instead of the symbol.
To learn more about density visit: https://brainly.com/question/24282743
#SPJ9
Points !!!!!!!!!!!!

Answers
Answer:
Point A, Point B, and Point C
Explanation:
Collinear points means that they share the same line. Points A, B, and C all share the line m.
High frequency wavelengths have _____wavelength?
varying
the same
high
low
Answers
The first part of the strontium test removes any residual barium. Do you have to be careful adding too much additional chromate? What might happen to the strontium ?
Answers
Yes, it is necessary to be careful when adding too much additional chromate during the strontium test. Excessive amounts of chromate can form a precipitate with strontium ions, leading to the formation of strontium chromate.
This can interfere with the accurate detection and measurement of strontium. Strontium chromate is a yellow solid that can precipitate out of the solution, making it difficult to distinguish and quantify the presence of strontium. This interferes with the accuracy and reliability of the strontium test. Therefore, it is important to use the appropriate amount of chromate in the test to ensure that the reaction specifically targets the barium ions without affecting the strontium ions.
Learn more about chromate here: brainly.com/question/28300460
#SPJ11
Why is an ecosystem still healthy although there's still a lot of consumers in it?
Answers
Answer:
because it is a sign that there are more producers for the consumers.
Explanation:
An ecosystem consist of the abiotic or inorganic components, the animals[consumers], the plants[producers] and the decomposers. An ecosystem simply means the environment of organisms that are living together and the way these organisms interact with another.
The producers that is the plants are the ones manufacturing food by themselves either by photosynthesis or chemosynthesis and the consumers are the ones relying on the producers for food.
The ecosystem is still healthy even when there's still a lot of consumers in it because it shows that there are come producers that is to say the producers are more also. If this is not the case, then the ecosystem will not be healthy as the consumers will eat the producers until the producers will not be enough.
Here is the electron configuration for Magnesium. How many total electrons are in the 2nd energy level?
Mg: 1s2 2s2 2p6 3s2
Answers
Answer:
8 electrons
Explanation:
Magnesium is present on group 2.
It has 2 valence electrons.
Electronic configuration of magnesium:
Mg₁₂ = 1s² 2s² 2p⁶ 3s²
1st energy level contain 2 electrons.(1s²)
2nd energy level contain 8 electrons. (2s² 2p⁶)
3rd energy level contain 2 electrons. (3s²)
3rs energy level of magnesium is called valence shell. It contain two valance electrons. Magnesium can easily donate its two valance electrons and get stable electronic configuration.
It react with halogens and form salt. For example,
Mg + Cl₂ → MgCl₂
art 5: Limiting Reagents
1. How many moles of water can be produced from 2.5mols H₂ and 4.5mols O₂?
2H₂ + O2 → 2H₂O
%657 16
What is the limiting reagent?
What is the excess reagent?
How many moles of water can be produced from this reaction?
Answers
Answer:To determine the amount of water that can be produced from the reaction of 2.5 moles of H₂ and 4.5 moles of O₂, we first need to determine the limiting reagent. The limiting reagent is the reactant that runs out first and limits the amount of product that can be formed. In this reaction, the limiting reagent would be the reactant with the lowest stoichiometric ratio to the product, in this case, O₂.
Since 4.5 moles of O₂ is present, and the reaction requires 2 moles of O₂ for every 2 moles of H₂, only 2.5 moles of water can be produced. This means that 2.5 moles of H₂ is the limiting reagent, and the 4.5 moles of O₂ is the excess reagent.
In this reaction, 2.5 moles of water can be produced from the reaction of 2.5 moles of H₂ and 4.5 moles of O₂.
Explanation:
Please help me with this!
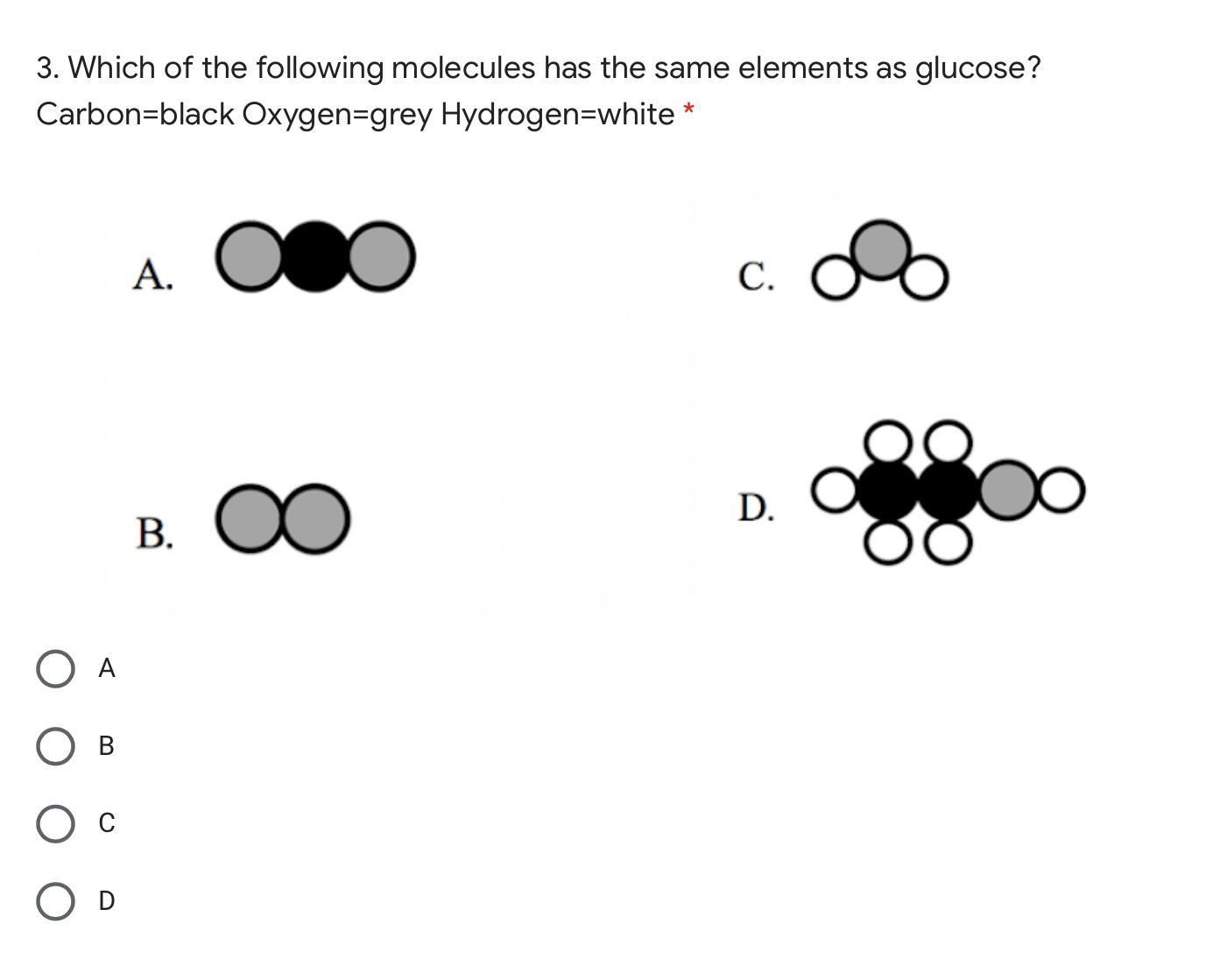
Answers
Answer:
D
Explanation:
Glucose is made of 6 Carbon atoms, 12 Hydrogen atoms, and 6 Oxygen atoms.
The equation is: C6H12O6
Answer: D
Explanation: D because it has more molecules elements then the glucose.
if 0.120 m hcl is slowly added to the naoh sample, in what ph range will the indicator change color?
Answers
If 0.120 m HCl is slowly added to the NaOH sample, then if the acidic solution increase greater than basic solution, the pH range indicator will change its color between 4.4 and 6.2 indicating red color.
Methyl red has a pKa value of 5. Consequently, between its pKa values of 4 and 6, it displays a colour change range of +/-1 pH unit. In acidic conditions, methyl red, an indicator dye, turns red. It serves as a pH indicator, turning yellow above pH 6.4 and orange between pH 4.4 and 6.2. An azo dye called methyl red is created from benzoic acid and a 2-positional 4-[(dimethylamino)phenyl]diazenyl group. It serves as a dye. It is a monocarboxylic acid, a tertiary amino compound, and a member of the azobenzene family.
To learn more about pH click here https://brainly.com/question/491373
#SPJ4
Neon gas at 305 k is confirmed within a constant volume at pressure p1. if the gas has a pressure p2 when it is cooled to 125 k, what is the ratio of p2 to p1?
Answers
Constant Volume pressure is also known as Isochoric Pressure.
Isochoric Pressure is defined as the thermodynamic process takes place at constant volume.
Simple Example of isochoric pressure is pressure cooker in this the volume of a cooker is constant.
Another name of isochoric process is isovolumetric process, or an isometric process.
According to question,
Given, T2 = 125k
T1 = 305k
V = const
PV = nRT
P/ T = const
P1/ T1 = P2/T2
We have to find the ration of T2/T1
P2/P1 = T2/ T1
= 125/305
= 25/61 or 0.4098
To know more about isochoric pressure here :
https://brainly.com/question/13261035?referrer=searchResults
#SPJ4
why do depositions often look layerd?
Answers
Sedimentary rocks have layers because of different depositions of sediments over time.
As the reaction in a galvanic cell proceeds towards products, which of the following are true?
A) ΔG starts at 0, stays same
B) ΔG starts < 0, becomes more negative
C) ΔG starts < 0, stays same
D) ΔG starts < 0, becomes more positive
E) ΔG starts > 0, stays same
Answers
In a galvanic cell, the reaction proceeds towards the production of products. ΔG starts < 0, becomes more negative
Option B is correct .
As the reaction proceeds, the Gibbs free energy (ΔG) reduces, and the following are true: ΔG starts < 0, becomes more negative.
When the reaction in a galvanic cell proceeds towards the production of products, the Gibbs free energy starts with a negative value, and it becomes even more negative.
The Gibbs free energy (ΔG) is a measure of the available energy in a system that can be used to do work. It measures the difference between the free energy of the final state and the initial state.The Gibbs free energy change of a system is dependent on the enthalpy and entropy change. If the enthalpy change is negative (exothermic), and the entropy change is positive (disorderly), the Gibbs free energy change is negative, and the reaction is spontaneous.
Learn more about Gibbs energy :
brainly.com/question/13765848
#SPJ11
So far, you have seen what happens when iodine is added to known chemicals. How do tests like these help scientists figure out information about unknown samples?
Answers
Tests can help the scientist to know the actual compound by interaction with some other compounds.
How can a scientist figure out information about unknown samples?Many times in the laboratory, we find that we come across samples that are unknown and it is important that the scientist should be able to figure out how we can be able to obtain the precise and accurate information that we need about the compound that is under study.
There are several ways that we can go about trying to find out the nature of a sample that is unknown. The most common way is to conduct a series of chemical reactions or to use various instrumental procedures in order to obtain the required information about the compound.
The way in which a compound interacts with reagents sheds light om what the composition of the compound would be and this is key in the identification of the compound.
Learn more about compounds:https://brainly.com/question/14658388
#SPJ1
what rule/principle states that electrons fill orbitals from lowest energy to highest enegery?
Answers
Answer:
The Aufbau Principle
Explanation:
In the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before occupying higher-energy levels.
Answer:
Aufbau principle
Explanation:
edge 2021
PLEASE ANSWER ASAP
Describe a procedure that could be used to separate a mixture of San and table salt.
Answers
Answer:
To separate sand and table salt you would add water. Table salt dissolves in water leaving salt water and sand. The sand then will go through the process of filtration leaving salt water and sand separated.
Explanation:
Perform the following operationand express the answer inscientific notation.8.6500x103 + 6.5500x105[? ]x10!?)

Answers
First, we need to make the exponent of 10 the same for both.
So let's transform 6.5500 x 10^5 into some number x 10^3.
For this, we need to move the dot to the right, some places where it gives the number 3. In this case, 2 places.
655.00 x 10^3
now we can sum the numbers
8.6500 x 10^3 + 655.00 x 10^3 = 663.65 x 10^3
now we need to transform this number into scientific notation. For this, must have only one number before the dot(on the left side of the dot). We will move the dot to the left, 2 places:
6.6365 x 10^5
Answer: 6.6365 x 10^5
Mac took an exceptional hard chemistry exam and is angry at his teacher. After the exam, Mac returns home and takes out his anger on his little brother. Mac's behavior illustrates a rationalization b displacement c reaction formation d regression e projection
Answers
Mac's behavior in the example provided illustrates projection.
What is Projection?Projection is defined as a mental process in which people attribute or
displace their feelings onto others. This is usually done unconsciously
under stressful conditions.
In this scenario, we were told that Mac was angry at his teacher and took
out his anger on his little brother which depicts Projection.
Read more about Projection here https://brainly.com/question/4523765
cehgg suppose 14.0 g of ice at -10.0c is placed into 300.0 g of water in a 200.0-g copper calorimeter. the final temperature of the water and copper calorimeter is 18.0c. 1) what was the initial common temperature of the water and copper? (express your answer to three significant figures.)
Answers
The initial temperature of the water and copper is 22.51°C.
Mass of ice m = 14 g
We know, 1g = 0.001 kg
So, m= (14 x 0.001)kg = 0.014kg
Mass of water ml = 300g = (300 x 0.001)kg = 0.3kg
Mass of copper calorimeter m₂ = 200g = (200 × 0.001) kg = 0.21 kg
Specific heat of water s₁ = 4186 \(\frac{J}{kg . C}\)
Specific heat of ice s₂ = 2100 \(\frac{J}{kg.C}\)
Specific heat of copper s₃ = 387 \(\frac{J}{kg.C}\)
Latent heat of fusion of water L = 3.33 x 10⁵ \(\frac{J}{kg}\)
Initial temperature of ice T₁ = - 10°C
Initial temperature of water copper is T₂
Final temperature of the system is T = 18 °C
From equilibrium in heat
Heat gain by ice to become 0°C of ice +0°C of ice become 0 °C of water + 0°C of water become 18°C of water = heat loss by copper and water
ms₂ (0°-(-10°)) + mL + ms₁ (T -0°) = m₁s₁ (T₂-18°) + m₂s₃ (T2 - 18°)
∴ 0.014 × 2100 × 10 + 0.014 × 3.33 × 10⁵+ 0.014 × 4186 × 18 = 0.3 × 4186 × (T₂- 18 ) + 0.2 × 387 × ( T₂ -18 )
∴ 6010.87 = 1333.2(T₂ - 18)
∴ T₂ - 18 = 6010.87 / 1333.2 = 4.51
∴ T₂ = ( 18 + 4.51 )
∴ T₂ = 22.51° C
So, the initial temperature of the water and copper is 22.51°C.
To learn more about Energy, Here :
https://brainly.com/question/29763173?referrer=searchResults
#SPJ4
the process of transforming Pyruvate and Acetyl CoA requires the use of 5 coenzymes. provide the name & structure of all these coenzymes below.
Answers
The process of transforming Pyruvate into Acetyl CoA involves the Pyruvate Dehydrogenase Complex, which requires the use of 5 coenzymes. These coenzymes are:
1. Thiamine pyrophosphate (TPP) - A coenzyme derived from vitamin B1 (thiamine) that is essential for the decarboxylation of pyruvate.
Structure: https://pubchem.ncbi.nlm.nih.gov/compound/Thiamine-diphosphate
2. Lipoamide - A coenzyme required for the transfer of acetyl groups during the reaction.
Structure: https://pubchem.ncbi.nlm.nih.gov/compound/Lipoamide
3. Coenzyme A (CoA) - A coenzyme derived from vitamin B5 (pantothenic acid) that is crucial for the formation of Acetyl CoA.
Structure: https://pubchem.ncbi.nlm.nih.gov/compound/Coenzyme-A
4. Nicotinamide adenine dinucleotide (NAD+) - A coenzyme derived from vitamin B3 (niacin) that acts as an electron carrier in the oxidation process.
Structure: https://pubchem.ncbi.nlm.nih.gov/compound/Nicotinamide-adenine-dinucleotide
5. Flavin adenine dinucleotide (FAD) - A coenzyme derived from vitamin B2 (riboflavin) that acts as an electron carrier and is required for the regeneration of lipoamide.
Structure: https://pubchem.ncbi.nlm.nih.gov/compound/Flavin-adenine-dinucleotide
These coenzymes work together to facilitate the transformation of Pyruvate into Acetyl CoA, a critical step in cellular respiration.
Visit here to learn more about coenzymes : https://brainly.com/question/29418914
#SPJ11
10. What illnesses or other damage to humans can be caused by UV light?
Answers
please help me
16 1 point What is the decay rate of a sample of Oxygen-21 if the sample has 8.31x1017 atoms and a decay constant of 0.203/s? 4.09x1018Bq 1.69x10¹7Bq 0.203Bq 2.44x10-1⁹Bq Previous
Answers
decay rate of approximately 1.69x10^17 Bq (becquerels),
The decay rate of a radioactive sample is determined by the number of radioactive atoms present and the decay constant, which represents the probability of decay per unit of time.
To calculate the decay rate, we multiply the number of atoms in the sample by the decay constant. In this case, the sample has 8.31x10^17 atoms and a decay constant of 0.203/s. Multiplying these values gives a decay rate of approximately 1.69x10^17 Bq (becquerels), which represents the number of decays per second in the sample.
Learn more about Oxygen here : brainly.com/question/13905823
#SPJ11
I need help, pls someone help me!!

Answers
Emma wants to go swimming the first day the pool opens in May, but she is worried it will be too cold since summer only just started. She only likes to swim in the water if the pool is at least 82°F. The thermometer in the pool reads 24°C. Is the pool warm enough for Emma to swim in? Show all work to support your answer.
Answers
Answer:
the water is not warm enough for her
Explanation:
Given that
Her preferred temperature = 82°F
Thermometer in water reads = 24°C
Convert the preferred temperature to °C
°C=5/9 (82-32)
°C = 27.8 °C
Hence the water is not warm enough for her
The water is not warm enough for her to swim in.
She loves to swim in water at temperature = 82°F.Thermometer in water reads = 24°C.We have to convert Emma's preferred temperature to °C
°C= 5/9 x (F - 32)
°C=5/9 (82-32)
°C = 27.8
Therefore the temperature is 27.8°C
This means that the temperature of the water is not warm enough for her
to swim in.
Read more about Temperature here https://brainly.com/question/1852859
