helpppp in a chemical equation, in which direction does the "yields" arrow point, and what factor does it indicate?
It points to the left, toward the reactants.
It points to the left, toward the products.
It points to the right, toward the reactants.
It points to the right, toward the products.
Answers
Answer:
It points right, towards the reactants
Explanation:
In a chemical equation, the arrow indicating the yield is to the right towards the arrow. The reactants are written in the left side and the product are written in the right side.
What is a chemical equation?A chemical equation represents the reaction between two or more elements or compound. The reaction describes the chemical combination between atoms.
A general chemical equation representing a chemical reaction is written below:
A + B ⇒ C.
Here, A and B are reactants and C be the product.
The yield of a reaction is the amount of product obtained. The theoretical yield might differ from the actual yield. Therefore, the arrow of a reaction points to the right towards products.
Find more on chemical equation:
https://brainly.com/question/30087623
#SPJ2
Related Questions
The mass of a solid substance is 21.112 g. If the volume of the solid substance is 19.5 cm3, calculate the density of the substance (in g/cm3) with the correct number of significant digits. The density is g/cm3.
Answers
Answer:
ρ = 1.08 g/cm³
Explanation:
Step 1: Given data
Mass of the substance (m): 21.112 g
Volume of the substance (V): 19.5 cm³
Step 2: Calculate the density of the substance
The density (ρ) of a substance is equal to its mass divided by its volume.
ρ = m / V
ρ = 21.112 g / 19.5 cm³
ρ = 1.08 g/cm³
The density of the substance is 1.08 g/cm³.
the valency of magnesium is 2 why
Answers
The valency of magnesium is 2 because it has two valence electrons in its outermost shell. Magnesium belongs to the second group of the periodic table, which means it has two electrons in its outermost shell or valence shell. In order to achieve a stable configuration, magnesium tends to lose these two valence electrons to form a positively charged ion with a charge of 2+. This makes magnesium a bivalent or divalent element with a valency of 2.
Answer:
Because the outer shell of magnesium contains 2 atoms
a society decides that it needs to produce more corn to feed the growing population what must it do before it begins the production process
Answers
The transformation of inputs into outputs is referred to as the production process.
What is meant by the production process?
A production process is a way to use economic resources, such as labor, expensive machinery, or land, to create goods and services for customers.
The transformation of inputs into outputs is referred to as the production process. The primary categories include mass, batch, job, just-in-time, and flexible manufacturing systems.
A production process is a method of delivering goods and services to clients while utilizing resources or economic inputs like labor, expensive machinery, or land.
Therefore, the correct answer is option A. analyze existing resources, such as water availability
The complete question is:
Society decides that it needs to produce more corn to feed the growing population. What must it do before it begins the production process?
A. analyze existing resources, such as water availability
B. determine how people will receive the surplus crops
C. study the profitability of increasing corn production
D. understand who is in a position of authority to produce more
To learn more about the production process refer to:
brainly.com/question/15240315
#SPJ13
Empriical formula for Na=29.1 S=40.5. O=30.4
Answers
Answer:
Na2S2O3.
Explanation:
What is given?
If we suppose that the compound has a mass of 100 g:
Mass of Na = 29.1 g,
Mass of S = 40.5 g,
Mass of O = 30.4 g,
Molar mass of Na = 23 g/mol,
Molar mass of S = 32 g/mol,
Molar mass of O = 16 g/mol.
Step-by-step solution:
First, we have to convert all the given masses of each element to moles using their respective molar mass, like this:
\(\begin{gathered} 29.1\text{ g Na}\cdot\frac{1\text{ mol Na}}{23\text{ g Na}}=1.27\text{ mol Na,} \\ \\ 40.5\text{ g S}\cdot\frac{1\text{ mol S}}{32\text{ g }}=1.27\text{ mol S,} \\ \\ 30.4\text{ g O}\cdot\frac{1\text{ mol O}}{16\text{ g O}}=1.9\text{ mol O.} \end{gathered}\)The next step is to divide each number of moles by the least number of moles obtained, which in this case is 1.27, as follows:
\(\begin{gathered} 1.27\text{ mol Na/1.27=1 mol Na,} \\ 1.27\text{ mol S/1.27=1 mol S,} \\ 1.9\text{ mol O/1.27=1.5 mol O.} \end{gathered}\)Remember that we need to have the number of moles of each element as integers, so if we multiply each number of moles of each element by 2, we will obtain:
\(\begin{gathered} 1\text{ mol Na}\cdot2=2\text{ mol Na,} \\ 1\text{ mol S}\cdot2=2\text{ mol S,} \\ 1.5\text{ mol O}\cdot2=3\text{ mol O.} \end{gathered}\)We would have 2 mol of Na, 2 mol of S, and 3 mol of O, so the empirical formula is Na2S2O3
Element Z has two naturally occurring Z-20 and Z-22. If the atomic mass of Z is 21.5 amu, what is the relative abundance of each isotope in nature.
Answers
The percentage abundance of the isotope Z-20 is 10.8 % and that of second isotope Z-22 will be 100 -10.8 = 89.2 %.
What is isotope?Isotopes are atoms of same atomic number and different mass number. An element can have two or more isotopes. The percentage abundance of each isotopes in the nature will be different.
Given that the average atomic mass of Z is 21.5 amu. It is the sum of products of the mass and abundance of each isotopes. Let x be the % abundance of Z-20 and 100-x for Z-22, now it can be written as:
20 x + 22 (100 - x ) = 21.5
20 x - 2200 -22x = 21.5
2x = 2200-21.5
x/100 = 10.8 %.
Therefore percentage abundance of Z-20 is 10.8 % and that of Z-22 is 100 - 10.8 = 89.2 %.
To find more on isotopes, refer here:
https://brainly.com/question/21536220
#SPJ1
A book is resting on a table. The mass of the book is 10 kg. The acceleration of gravity
is 9.8 m/s2. Calculate the force the book exerts on the table.
Answers
Answer:
98N
Explanation:
fg=mg
=(10)(9.8)
=98N
The sun provides _____ to a plant, when a person eats the plant, ____ is released and then transforms into___ when the person goes for a run
Answers
Answer: no answer
Explanation:
Trees often lose their leaves in the winter. Explain what this tree will not be able to do without its leaves.
Answers
Answer:
Most aren't able to take in carbon dioxide gas from the air or produce any oxygen.
Explanation:
science bro
7. In the process of assembling bicycles, you have 114 frames, 300 tires, 75 seats, 109 sets of pedals, and 84 sets of handlebars. Which is the limiting reactant in this process?
a. frames
c. seats
e. handlebars
b. tires
d. pedals
Answers
In the bicycle assembling process, the limiting reactant is seats since they will be used up first.
What is a limiting reactant?A limiting reactant is a reactant which is used up first in a reaction and on which product formation depends on.
In a given reaction, once the limiting reactant is used up, the reaction will stop.
For a bicycle to be assembled, 1 frame, 1 seat, 1 seat of handlebars, 1 seat of pedals and 2 tires are required.
In the process of assembling bicycles, there are 114 frames, 300 tires, 75 seats, 109 sets of pedals, and 84 sets of handlebars.
Therefore, the limiting reactant is seats since they will be used up first.
Learn more about limiting reactant at: https://brainly.com/question/14225536
#SPJ1
A piece of unknown metal weighs 500 g. When the piece of metal absorbs 6.64 kJ of heat, its temperature increases from 25°C to 78°C. What's the specific heat capacityof this metal?A. 13 J/gºCB. 0.25 J/gºCC. 25 J/gºCD.2.5 J/g °C
Answers
0.25 J/gºC
Explanations:The formula for calculating the heat absorbed by the metal is expressed as:
\(Q=mc\triangle t\)where;
m is the mass of the object
c is the specific heat capacity
△t is the change in temperature
Given the following parameters
m = 500g
△t = 78 - 25 = 53°C
Q = 6.64kJ = 6640Joules
Substitute
\(\begin{gathered} 6640=500c(53) \\ c=\frac{6640}{500\times53} \\ c=\frac{6640}{26500} \\ c=\frac{0.25J}{g^oC} \end{gathered}\)Therefore the specific heat capacity of this metal is 0.25 J/gºC
What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
Ordered: 0.3 gm. Available: 300 mg/tablet. How many tablets should be given?
Answers
Answer:
1 tablet
Explanation:
Assuming that gm stands for gram:
Put everything in one unit, 0.3 g = 300 mg.
And given that each tablet is 300 mg:
mass/amount = 300mg / 1 tablet
300mg / X amount = 300mg / 1 tablet
(300mg/300mg) tablet = X = 1 tablet
So it is 1 tablet you need.
One tablet should be given. A dosage is a set quantity of medication administered all at once.
What is dosage?A dosage is a set quantity of medication administered all at once. Contrarily, the dosage is the recommended way to take the drug: a specified quantity, amount, and regularity of doses spread over a specific amount of time.
To put it another way, a dosage is simply the quantity of medication that you consume at one particular moment. The dosage consists of the pharmacological dose, or amount, as well as the timing and frequency of administration. How you should administer or take the recommended medication is determined on the dosage.
0.3 g = 300 mg.
each tablet is 300 mg:
mass/amount = 300mg / 1 tablet
300mg / X amount = 300mg / 1 tablet
(300mg/300mg) tablet = X = 1 tablet
Therefore, one tablet should be given.
To know more about dosage, here:
https://brainly.com/question/4459950
#SPJ2
Which is the state of matter that has an indefinite shape and indefinite volume?
Answers
Answer:
gas
Explanation:
gas: indefinite shape, indefinite volume. Indefinite shape means that the sample in question takes on the shape of the container.
Which of the following results when hydrogen and chlorine chemically bond to form hydrogen chloride?
A.
a solution
B.
a mixture
C.
a precipitate
D.
a compound
Answers
Answer:
Hydrogen chloride is a mixture of hydrogen and chlorine elements. The chemical abbreviation of hydrochloric acid is HCl.
Explanation:
Successful rifting can create new oceans and split apart continents. True or False
Answers
Answer:
True if by rifting you mean continental rift. If not idk.
Submit your organization chart about the fictional elements. Remember to proofread your work before submitting.
Answers
Answer:
follow the picture,,, it includes the normal elements too
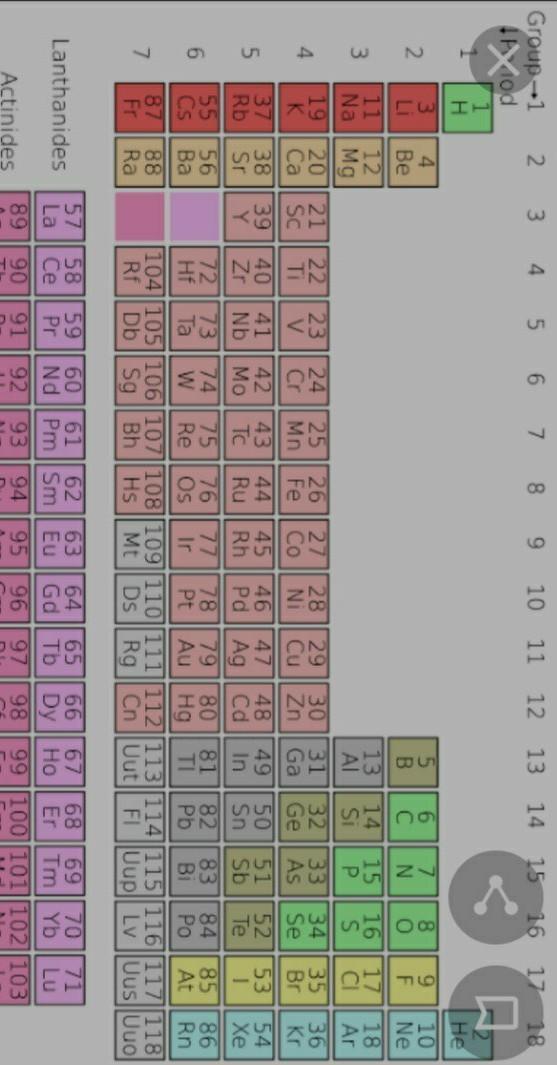
The reason why I organized these specific elements from the list of elements that you provided me is because they have to do with color. I believe organizing by color would be much easier for me to understand. There is also a somewhat equal amount for each color. For example I used the colors: orange, green and red In my chart because there are four of each color in the list you provided me.
There are 85.63 g of CO2 gas in a container maintained at STP. How many liters of CO2 are there in that container?
Answers
Answer:
V = 48.64 L
Explanation:
Given data:
Mass of CO₂ = 85.63 g
Temperature = 273 K
Pressure = 1 atm
Volume of CO₂ = ?
Solution:
Number of moles of CO₂:
Number of moles = mass/molar mass
Number of moles = 85.63 g / 40 g/mol
Number of moles = 2.14 mol
Volume in Litter:
PV = nRT
R = general gas constant = 0.0821 atm.L/mol.K
1 atm× V = 2.14 mol ×0.0821 atm.L/mol.K ×273 K
V = 48.64 atm.L / 1 atm
V = 48.64 L
Calculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
What medal has the highest volume?
Answers
The Medal of Honor is the best navy decoration and highest volume.
The President of the USA The Victoria go is the holy grail for Medal of Honor creditors because there are the best in lifestyles. Bearing the inscription For valor and called a VC, this medal turned into first offered for conspicuous bravery' in 1856 and later backdated to the Crimean conflict of 1854. The outstanding service pass is the second maximum army ornament that may be provided to a member of the American military, for intense gallantry and risk of lifestyle in actual combat with an armed enemy force.
The Bronze Star Medal dates lower back to world battle II. these days, it is the fourth-maximum ranking award a provider member can obtain for a heroic and meritorious deed performed in an armed battle. For individuals who acquire the BSM, it's far a sign of their sacrifice, bravery, and honor at the same time as serving their us of a
Learn more about medals here:-https://brainly.com/question/17634999
#SPJ9
An infant acetaminophen suspension contains 80 mg/0.80 mL suspension. The recommended dose is 15 mg/kg body weight.
How many milliliters of this suspension should be given to an infant weighing 13 lb.
Answers
Answer:
0.8853 mL
Explanation:
First we convert 13 lb to kg, keeping in mind that 1 lb = 0.454 kg:
13 lb * \(\frac{0.454kg}{1lb}\) = 5.902 kgThen we calculate how many mg of acetaminophen should be given, using the recommended dose and infant mass:
15 mg/kg * 5.902 kg = 88.53 mgFinally we calculate the required mL of suspension, using its concentration:
88.53 mg ÷ (80 mg/0.80 mL) = 0.8853 mLhow many grams of N2 are needed to make 5.8g of N2O
Answers
First, we need the reaction:
2N2 (g) + O2 (g) → 2N2O (g)
Molecular weights:
N2 = 28.01 g/mol
N2O = 44.01 g/mol
For this exercise we use Stoichiometry.
Always balance your reaction.
---------------------------------------------------------------------------------
We know that:
2 x 28.01 g N2 -------------------- 2 x 44.01 g N2O
X -------------------- 5.80 g N2O
\(X\text{ = }\frac{5.8\text{ g x 2 x 28.01 g}}{2\text{ x 44.01 g}}=\text{ 3.69 g N2}\)Answer: 3.69 grams of N2 are needed.
When an atom loses energy by electron transition, a _______________ is produced.
A. Photon
B. wave
C. ray
D. Gamma particle
Answers
Answer:
Explanation:
If an electron is in an excited state it can return to a lower energy level. When it does this, it loses energy. The amount of energy it loses will be equal to the difference in the energy levels it moves between. This energy is released as a photon.
If 25.8 mL of an AgNO3 solution is needed to precipitate all Cl- ions in a 1570 mg of KCl (forming AgCl), what is the molarity of the AgNO3nsolution?
Answers
Answer:
M=0.816M
Explanation:
Hello,
In this case, we should consider the following reaction:
\(AgNO_3+KCl\rightarrow KNO_3+AgCl\)
Thus, by knowing the 1:1 molar ratio of silver nitrate and potassium chloride, we can easily compute the moles of silver nitrate precipitating the 1570 mg of potassium chloride considering its molar mass of 74.5513 g/mol:
\(n_{AgNO_3}=1570mgKCl*\frac{1gKCl}{1000mgKCl} *\frac{1molKCl}{74.5513gKCl}*\frac{1molAgNO_3}{1molKCl} \\\\n_{AgNO_3}=0.021molAgNO_3\)
Then, by using the volume of silver nitrate in liters (0.0258 L), we can directly compute the molarity:
\(M=\frac{0.021molAgNO_3}{0.0258L}\\ \\M=0.816M\)
Regards.
CHEM FINAL TOMORROW!!! I'm struggling with a few concepts, if anyone could help explain this to me & how to do it, I'd be very grateful!!!

Answers
Based on the given reaction, the acid-base pairs in this reaction are:
HCO₃⁻ (acid) and NH₃ (base)NH₄⁺ (acid) and CO₃²⁻ (base)What are the acid-base pairs in the given reaction?An acid-base pair refers to a set of two chemical species that are related through the transfer of a proton (H+ ion) during a chemical reaction.
One species acts as an acid by donating a proton, while the other acts as a base by accepting that proton.
In the given reaction:
HCO₃⁻ (aq) + NH₃ (aq) → NH₄⁺ + CO₃²⁻
An acid-base pair can be identified as follows:
HCO₃⁻ (bicarbonate ion) can act as an acid by donating a proton (H⁺), becoming CO₃⁻.
NH₃ (ammonia) can act as a base by accepting a proton (H⁺), becoming NH₄⁺ (ammonium ion).
Learn more about acid-base pairs at: https://brainly.com/question/22514615
#SPJ1
Need your help ASAP, please!
What is the molarity of a 4.9% H2SO4 solution of density 0.98 gm/ml?
The answer is 0.49 M, please do explain how
Answers
0.49 M is the molarity of the 4.9% \(H_{2}SO_{4}\) solution.
To find the molarity of a solution, we need to calculate the number of moles of the solute (\(H_{2}SO_{4}\)) present in a given volume of the solution. Here's how to determine the molarity of a 4.9% \(H_{2}SO_{4}\)solution with a density of 0.98 g/ml:
Determine the mass of the solute: The 4.9% concentration implies that 4.9 g of \(H_{2}SO_{4}\) is present in 100 g of the solution.
Calculate the volume of the solution: Divide the mass of the solution by its density. In this case, 100 g / 0.98 g/ml = 102.04 ml.
Convert the volume of the solution to liters: Divide the volume by 1000 to convert from milliliters to liters. 102.04 ml / 1000 = 0.10204 L.
Calculate the number of moles: Multiply the mass of the solute by its molar mass. The molar mass of \(H_{2}SO_{4}\) is 98.09 g/mol. Therefore, 4.9 g / 98.09 g/mol = 0.0499 mol.
Calculate the molarity: Divide the number of moles by the volume of the solution in liters. 0.0499 mol / 0.10204 L ≈ 0.49 M.
Thus, the molarity of the 4.9% \(H_{2}SO_{4}\) solution is approximately 0.49 M.
Know more about molarity here:
https://brainly.com/question/30404105
#SPJ8
What is the balanced formula for hydro phosphoric acid
Answers
Hydrophosphoric acid is generally known as phosphine and the formula for hydrophosphoric acid is PH3 P H 3
What is hydrophosphoric acid?Hypophosphoric acid is a mineral acid with phosphorus in an oxidation state +4.It has a chemical formula H4P2O6.
Hypophosphorous acid is used as a decolorizing agent and for color stabilization during the manufacture of chemicals and several plastics including: nylon fibers, polyamides, polyester fiber, polyacrilonitrile, alkyd rsins, epoxies, fatty acid esters and glycerols.
Therefore, the formula of hypophosphoric acid is H4P2O6. It contains four P-OH bonds, Two P=O. bonds and one P-P bond.
Learn more about hydrophosphoric acid here: brainly.com/question/21172804
#SPJ1
What is uranium classified as
Akali Metals, Akaline Metals, Metalloids, Halogen, Noble Gas, Transition Metal, Rare Earth Metal or Non-Metal?
Answers
Explanation:
Uranium is classified as a transition metals.
An clement X has 2 electrons in K shell, 8 electrons in L shell and 5 electrons in i Size of X ion is greater than that of X atom though both contain the same protons. Give reason. ii) Write down the formula of one of the compounds of X where X is in -3 oxidation.
Answers
Answer:
i) The size of X ion is greater than that of X atom even though both contain the same number of protons because the ion has fewer electrons compared to the atom. When an atom forms an anion (negative ion), it gains electrons, which causes increased electron-electron repulsion. This repulsion causes the electron cloud to expand, and as a result, the ion becomes larger than the neutral atom.
In the case of element X, when it forms an ion with a -3 charge, it will gain 3 more electrons, increasing the total number of electrons to 18. This will cause the size of the X ion to be larger than the neutral X atom.
ii) To determine the compound of X in the -3 oxidation state, we first need to determine the element's identity. We know that X has 15 electrons in total (2 in the K shell, 8 in the L shell, and 5 in the M shell). Therefore, X has an atomic number of 15, which corresponds to phosphorus (P).
Since phosphorus is in the -3 oxidation state, it gains 3 electrons and becomes P^3-. To form a compound, we need a cation that can balance the negative charge. A common example is aluminum (Al), which has a +3 charge (Al^3+). When phosphorus and aluminum combine, they form the compound aluminum phosphide with the formula AlP.
Name two indicators used in quantitative analysis.
Answers
1. Standard deviation: It measures the variation or volatility of the data points in a dataset. It is calculated by taking the square root of the variance. The higher the standard deviation, the more dispersed the data points are from the average. In finance, standard deviation is often used to measure the risk associated with investments. Higher standard deviation indicates higher risk.
2. Correlation coefficient: It measures the strength of the relationship between two variables. It is calculated by dividing the covariance between the two variables by the product of their standard deviations. Correlation coefficient can range from -1 to 1, where -1 indicates a perfect negative correlation, 0 indicates no correlation, and 1 indicates a perfect positive correlation. Correlation coefficient is often used in finance to assess the relationship between two stocks or other financial instruments. A higher correlation coefficient indicates a stronger relationship between the variables being compared.
Hope this helps? :)
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8