How did mendeleev come up with the first periodic table of the elements?.
Answers
Mendeleev, a Russian chemist, created the first periodic table of elements by arranging them in order of increasing atomic weight, with elements of similar properties occurring at regular intervals.
Mendeleev created the first periodic table of elements. He came up with the periodic table by arranging the elements in order of increasing atomic weight, with elements of similar properties occurring at regular intervals. Mendeleev was able to accurately predict the properties of some undiscovered elements, such as gallium, germanium, and scandium, using this periodicity. He was able to accurately predict the properties of some undiscovered elements using this periodicity.
Mendeleev's periodic table was the first to accurately predict the properties of unknown elements, and it is still used today as a basis for modern periodic tables. By placing the elements in order of increasing atomic weight, Mendeleev created a useful tool for organizing the known elements and making predictions about those yet to be discovered.
To know more about periodic table visit
https://brainly.com/question/28747247
#SPJ11
Related Questions
what is other name of molecules
Answers
Answer:molecule(noun) (physics and chemistry) the simplest structural unit of an element or compound. Synonyms: mote, atom, speck, corpuscle, particle. atom, molecule, particle, corpuscle, mote, speck(noun)
Explanation:
Pls help me I don’t know how to do this

Answers
Explanation:
We have a 63.9 g sample of calcium hydroxide. First we have to convert those grams into moles. To do that we have to use the molar mass of calcium hydroxide.
Calcium hydroxide = Ca(OH)₂
molar mass of Ca = 40.08 g/mol
molar mass of O = 16.00 g/mol
molar mass of H = 1.01 g/mol
molar mass of Ca(OH)₂ = 1 * 40.08 g/mol + 2 * 16.00 g/mol + 2 * 1.01 g/mol
molar mass of Ca(OH)₂ = 74.10 g/mol
mass of Ca(OH)₂ = 63.9 g
moles of Ca(OH)₂ = 63.9 g /(74.10 g/mol)
moles of Ca(OH)₂ = 0.862 moles
In 1 molecule of Ca we have 2 atoms of O. So in 1 mol of Ca(OH)₂ we will have 2 moles of O atoms.
1 mol of Ca(OH)₂ = 2 moles of O atoms
moles of O atoms = 0.862 moles of Ca(OH)₂ * 2 moles of O /1 mol of Ca(OH)₂
moles of O atoms = 1.724 moles
One mol is similar to a dozen. When we say that we need a dozen eggs we know that we need 12 eggs. If we want a mol of eggs, we want 6.022*10^23 eggs. So one mol of something is 6.022 * 10^23 of that.
1 mol of O atoms = 6.022 * 10^23 atoms
n° of O atoms = 1.724 moles * 6.022 * 10^23 atoms/1 mol
n° of O atoms = 1.04 * 10^24 atoms
Answer: In a 63.9 g sample of Ca(OH)₂ we have 1.04 *10^24 atoms of oxygen.
Purchase process: (50) The process begins with a department admin sending a purchase request to the IT department. The IT Manager reviews the request and if approved, requests a quote from Apple, Dell, HP, ASUS and Lenovo. If rejected, the request is sent back to the admin for review and has to be resubmitted to the IT Manager. The best price will be sent to the admin and once approved, the IT manager finalizes the vendor and then prepares the purchase request. The Procurement Supervisor receives the request and issues the purchase order to the vendor. The Procurement Supervisor then reviews the invoice and processing time from the vendor. By the end of the processing time, if the tracking number was not received, the Supervisor cancels the order. If vendor provides the tracking number, Procurement Supervisor collects the product once delivered and simultaneously submits the payment. Once both the steps are done, the process ends as the purchase is completed.
Answers
The purchase process involves steps such as initiating a request, vendor selection, approval, purchase order issuance, product delivery, and payment, ensuring a systematic approach to procurement for accountability and efficiency.
The purchase process consists of several steps:
1. The department admin initiates the process by sending a purchase request to the IT department.
2. The IT Manager reviews the request and decides whether to approve or reject it.
3. If the request is approved, the IT Manager contacts various vendors, such as Apple, Dell, HP, ASUS, and Lenovo, to request quotes.
4. The IT Manager receives the quotes and selects the best price.
5. Once the best price is selected, the IT Manager informs the admin and waits for their approval.
6. If the admin approves, the IT Manager finalizes the vendor selection and prepares the purchase request.
7. The IT Manager then sends the purchase request to the Procurement Supervisor.
8. The Procurement Supervisor receives the request and issues a purchase order to the chosen vendor.
9. The Procurement Supervisor reviews the vendor's invoice and processing time.
10. If the processing time elapses and the tracking number has not been received, the Procurement Supervisor cancels the order.
11. If the vendor provides the tracking number within the processing time, the Procurement Supervisor collects the product once it is delivered.
12. At the same time, the Procurement Supervisor submits the payment to the vendor.
13. Once both steps are completed, the purchase process is considered finished, and the purchase is completed.
This process ensures that there is a clear and systematic approach to purchasing items, from the initial request to the final delivery and payment. Each step is important in maintaining accountability and efficiency in the procurement process.
Learn more about accountability here :-
https://brainly.com/question/29108212
#SPJ11
Some athletes like runners or sprinters may train by attaching a small parachute to their upper body. The parachute opens behind them as they run, resulting in a drag force acting on the athlete. Such athletes would never use this in actual competition, though. Explain how using a parachute like this might make an athlete stronger and why they wouldn’t actually use the parachute during a competition even though they trained with it.
Answers
Answer:
when using would make an athlete stronger when they keep on training with it.
they don't actually use it during a competition because it slows them down and they won't be able to perform well during Competition even though they trained with it
Explain the similarities and differences between putting a beaker of ethanoic acid in the refrigerator and mixing it with sodium carbonate
Answers
Answer:
See explanation
Explanation:
When a beaker of ethanoic acid is placed in the refrigerator, its temperature drops and the vessel feels cool.
Now, when we mix ethanoic acid and sodium carbonate, an endothermic reaction occurs, fizzing is observed as carbon dioxide is given off and heat is lost to the surroundings causing the reaction vessel to feel cool to touch.
The difference between putting ethanoic acid in the refrigerator and adding sodium carbonate to the solution is that, in the former, no new substance is formed. The substance remains ethanoic acid when retrieved from the refrigerator. In the later case, new substances are formed. The substance is no more ethanoic acid because a chemical reaction has taken place.
What is Gay- Lussacs law? State the definition of law in your own words.
Answers
describe a method to investigate how the temperature changes when different masses of ammonium
Answers
Answer:
Decrease temperature with increase in ammonium nitrate concentration.
Explanation:
The temperature changes when different masses of ammonium nitrate are dissolved in water because the ammonium nitrate takes energy from the surrounding water environment for the breaking of ionic bonds as a result the temperature of water decreases. When more concentration of ammonium nitrate is added to water, more decrease occur in temperature of water and the water becomes cold.
Calculate the volume in L of 11.6 moles of Neon at 120 K when it has a pressure of 25.9 atm
Answers
Answer:
The volume of the gas is approximately 4.41 liters
Explanation:
The details of the data of the Neon gas are;
The number of moles of Neon gas present, n = 11.6 moles
The temperature of the sample of Neon gas, T = 120 K
The pressure of the sample of the Neon gas, P = 25.6 atm
By the ideal gas equation, we have;
P·V = n·R·T
Where;
R = The universal gal constant = 0.08205 L·atm·mol⁻¹·K⁻¹
Therefore, we get;
V = n·R·T/P
Which gives;
V = 11.6 moles × 0.08205 L·atm·mol⁻¹·K⁻¹ × 120 K/(25.9 atm) ≈ 4.4097915 L
The volume of the gas, V ≈ 4.41 L.
Two tennis balls are dropped at the same time. One tennis ball is dropped from the roof of a two-story house. The other tennis ball is dropped from 1 m off the ground. Which tennis ball will be moving the slowest as it hits the ground? Why?
Answers
A sample of gas is placed in a rigid container. If the original conditions were 320 torr and 400 K, what will be the pressure in the container at 200 K?
a. 160 torr
b. 640 torr
c. 250 torr
d. 760 torr
Answers
P₁V₁/T₁ = P₂V₂/T₂
where P₁ and T₁ are the initial pressure and temperature, P₂ and T₂ are the final pressure and temperature, and V₁ and V₂ are the initial and final volumes (assuming constant volume in this case since the container is rigid).
Let's plug in the values given:
P₁ = 320 torr
T₁ = 400 K
T₂ = 200 K
Since the volume is constant, V₁ = V₂, so we don't need to include it in the equation.
Now, we can solve for P₂:
P₁/T₁ = P₂/T₂
P₂ = (P₁ * T₂) / T₁
= (320 torr * 200 K) / 400 K
= 160 torr
Therefore, the pressure in the container at 200 K would be 160 torr (option a).
HELP!!!!!!!!!!!
How is it possible that 2 objects have the same densities but different mass and volumes?
Answers
Answer:
So yes, two objects of the same substance, but with a difference size and/or shape can have the same density, it all comes down to the weight, the volume and the temperature to determine your density, that is assuming your object isn't hollow of course.
Explanation:
Answer:
it is possible
Explanation:
The density of a substance is the relationship between the mass of the substance and how much space it takes up (volume). Density equals the mass of the substance divided by its volume; D = m/v. Objects with the same volume but different mass have different densities.
Which atom (magnesium or chlorine) is larger? _______________________(you should also be prepared to answer the question if asked for the smaller atom)3a. Explain why the atom is larger. Include the following terms in your answer: protons, electrons, shells or layers, columbic attractions
Answers
Answer:
The magnesium atom is larger.
Explanation:
The magnesium atom is larger because it is on the left side of the Periodic Table (period 3 and group 2) where the atomic radius is larger.
Atomic radius is the distance from the center of the nucleus of an atom to the most external electron shell.
The greater the columbic attractions, the closer the protons in the nucleus are to the electrons in the outer layers, making the size of the atom smaller.
true or false a neutral atom must have the same number of neutrons and protons
Answers
Benzene has the chemical formula C6H6. Which of the statements below about benzene is TRUE?
Hints
A. The empirical formula of benzene is CH.
B. The molecular formula of benzene is CH.
C. The molecular formula of benzene is C12H12.
D. The molecular and empirical formulas for benzene are the same.
Answers
The true statement about benzene is D. The molecular and empirical formulas for benzene are the same.
The statement that is true about benzene is that the molecular and empirical formulas for benzene are the same. Benzene's chemical formula is C₆H₆, which represents its molecular formula. The molecular formula gives the actual number of atoms of each element in a molecule. In this case, benzene consists of six carbon atoms (C₆) and six hydrogen atoms (H₆).
The empirical formula, on the other hand, represents the simplest, most reduced ratio of elements in a compound. To obtain the empirical formula, one must divide the subscripts by their greatest common divisor. In the case of benzene, both the carbon and hydrogen subscripts are already at their simplest form. Thus, the empirical formula of benzene is the same as its molecular formula, which is C₆H₆.
Learn more about benzene here, https://brainly.com/question/6028840
#SPJ11
What happens to the heat energy that leaves the milk?
Answers
Answer:
it gets cold or warm
Explanation:
pleas get me 53 likes
Ok
Element symbol _____?
Total electrons _____?
valence electrons _____?
Tysm :D has

Answers
Explanation:
element symbol is Na
total electrons is 11
valences electron is 1
What is the mass of 2.40 mol H2O?
Answer in units of g.
Answers
Answer:
204
Explanation:
In acidic solution. A 70.0 mL sample of a solution containing Fe2+ requires 70.0 mL of a 0.150 M KMnO4 solution for complete reaction. What is the concentration of the Fe2+ in the original solution?
Answers
Answer:
0.75M Fe²⁺
Explanation:
First, we need to balance the redox reaction in acidic medium. Then, we can obtain moles of KMnO4 and with the reaction moles and molarity of the Fe²⁺ solution:
Redox Balance:
Fe²⁺ → Fe³⁺ + 1e⁻
5e⁻ + 8H⁺ + MnO₄⁻ → Mn²⁺ + 4H₂O
___________________________
5Fe²⁺ + 5e⁻ + 8H⁺ + MnO₄⁻ → 5Fe³⁺ + 5e⁻ + Mn²⁺ + 4H₂O
5Fe²⁺ + 8H⁺ + MnO₄⁻ → 5Fe³⁺ + Mn²⁺ + 4H₂OMoles of KMnO₄:
70.0mL = 0.0700L * (0.150mol / L) = 0.0105 moles KMnO₄
Moles and molarity Fe²⁺:
0.0105 moles KMnO₄ * (5 moles Fe²⁺ / 1mol KMnO₄) = 0.0525 moles Fe²⁺
In 70.0mL = 0.0700L:
0.0525 moles Fe²⁺ / 0.0700L =
0.75M Fe²⁺(Help Me Please!)
A student collected the data shown above. Row A may represent error
B
C
D
E
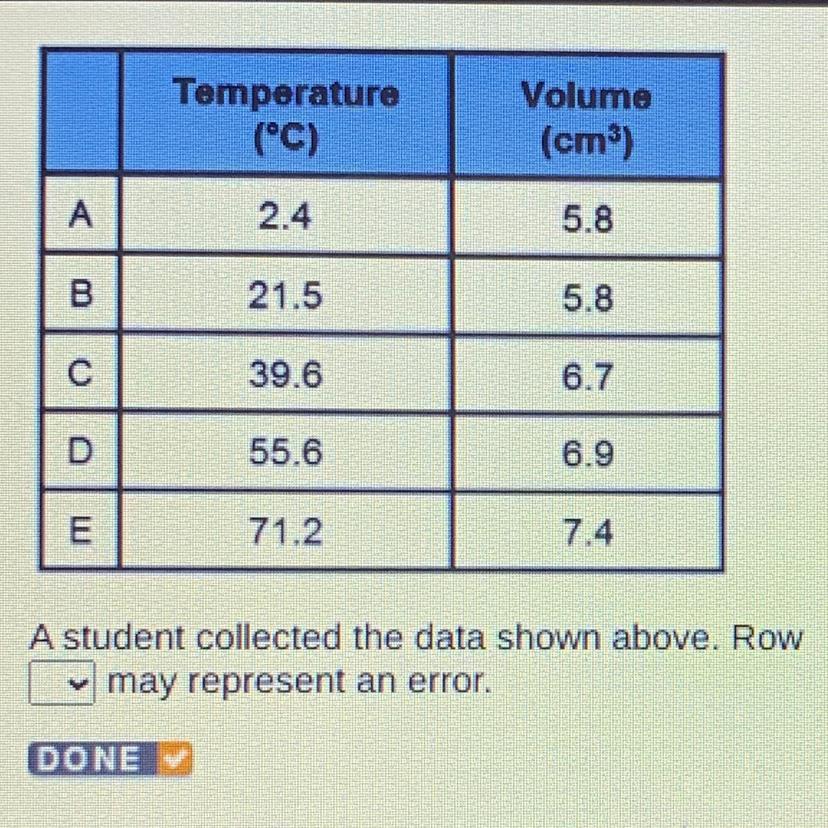
Answers
It’s the biggest diff of temp without volume
MULTIPLE CHOICE
True or False: Any metal object that contains iron,
such as steel, will be attracted to a magnet.

Answers
Answer:
TRUE
Explanation:
A small amount of bleach was accidentally spilled on a kitchen floor. After a while it was
observed that the floor appeared to be dry and the whole kitchen smelled of bleach.
Which processes have occurred?
A
Evaporation and diffusion
B
Distillation and diffusion
с
Evaporation only
D
Evaporation and Brownian motion
Answers
Mixtures:
(A) Have specific compositions
(B) do not have specific compositions
(C) are made from liquids
(D) are made from one substance
Answers
The true statement about mixtures is that they do not have specific compositions (option B).
What is a mixture?Mixture is a substance made up of two or more chemical components that are not chemically linked.
This further means that the constituents of a mixture maintain or keep their individual chemical identities because there is no breaking of bonds.
As opposed to mixtures, compounds are made up of constituents that are chemically bonded to one another, hence, possess a definite composition.
Examples of mixtures are as follows:
Sugar and waterSalt and waterAir (mixture of gases)Salt and sugarSand and waterOil and waterTherefore, the true statement about mixtures is that they do not have specific compositions.
Learn more about mixtures at: https://brainly.com/question/24898889
#SPJ1
How much iodine should you take if exposed to radiation.
Answers
Answer:
12-18 65mg or sometimes 130mg if its just one or 1/2 a tablet
Explanation:
The equilibrium constant Kc for the reaction
N2 (g) + 3H2 (g) -> 2NH3 (g)
at 450°C is 0.159. Calculate the equilibrium composition
when 1.00 mol N2 is mixed with 3.00 mol H2 in a 2.00-L
vessel.
part 1: Enter the equilibrium concentration for N2.
part 2: Enter the equilibrium concentration for H2
part 3: Enter the equilibrium concentration for NH3.
Answers
Answer:
[N2] = 0.3633M
[H2] = 1.090M
[NH3] = 0.2734M
Explanation:
Based on the reaction of the problem, Kc is defined as:
Kc = 0.159 = [NH3]² / [N2] [H2]³
Where [] are the equilibrium concentrations.
The initial concentrations of the reactants is:
N2 = 1.00mol / 2.00L = 0.500M
H2 = 3.00mol / 2.00L = 1.50M
When the equilibrium is reached, the concentrations are:
[N2] = 0.500M - X
[H2] = 1.50M - 3X
[NH3] = 2X
Where X is reaction quotient
Replacing in the Kc equation:
0.159 = [2X]² / [0.500 - X] [1.50 - 3X]³
0.159 = 4X² / 1.6875 - 13.5 X + 40.5 X² - 54 X³ + 27 X⁴
0.268313 - 2.1465 X + 6.4395 X² - 8.586 X³ + 4.293 X⁴ = 4X²
0.268313 - 2.1465 X + 2.4395 X² - 8.586 X³ + 4.293 X⁴ = 0
Solving for X:
X = 0.1367. Right solution.
X = 1.8286. False solution. Produce negative concentrations
Replacing:
[N2] = 0.500M - 0.1367M
[H2] = 1.50M - 3*0.1367M
[NH3] = 2*0.1367M
The equilibrium concentrations are:
[N2] = 0.3633M[H2] = 1.090M[NH3] = 0.2734MSEP Develop a Model Write the skeleton equation for the reaction of iron and diatomic oxygen gas to form iron (III) oxide (Fe2O3). Sketch a molecular model of the reactants and products in the reaction.
Urgent
Answers
A balanced equation obey the law of conservation of mass. According to this law, the mass can neither be created nor be destroyed but it can be converted from one form to another. A skeleton equation is the balanced equation.
A chemical equation in which the number of atoms of reactants and products are equal on both sides of the equation is defined as the balanced equation. The numbers which are used to balance the chemical equation are called the coefficients.
The skeleton equation between diatomic oxygen and iron (III) oxide is:
4Fe (g) + 3O₂ (g) → 2Fe₂O₃ (g)
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
A series of cell voltages are measured from an electrochemical cell constructed with Zn anode in 1.00 M ZnSO4 solution and Ag cathode in various standard solutions of AgNO3 from 0.0001 M to 0.1000 M. A plot of measured Ecell is plotted as function log10[Ag ] for the cell with the different concentrations of AgNO3. Predict the theoretical value of the slope of the plot
0.05916 V
0.02958 V
0.02569 V
0.01284 V
0.01690V
Predict the theoretical value of the y-intercept.
2.362 V
–1.562 V
0.455 V
–0.455 V
1.562V
Answers
The theoretical value of the slope of the plot is 0.02958 V, and the theoretical value of the y-intercept is 0.455 V.
In electrochemical cells, the measured cell potential (Ecell) can be related to the concentrations of the reactants using the Nernst equation:
Ecell = E°cell - (0.05916 V/n) * log10([Ag⁺]/[Zn₂⁺])
where E°cell is the standard cell potential, n is the number of electrons transferred in the cell reaction, [Ag⁺] is the concentration of silver ions, and [Zn₂⁺] is the concentration of zinc ions.
In this case, the anode is constructed with a zinc electrode in a 1.00 M ZnSO₄ solution, and the cathode is made of silver in various standard solutions of AgNO₃ with concentrations ranging from 0.0001 M to 0.1000 M. The plot of Ecell versus log10[Ag⁺] will give us insights into the relationship between the cell potential and the concentration of silver ions.
The slope of the plot represents the value of (0.05916 V/n), which is a constant. By observing the given choices, the closest value to 0.05916 V is 0.02958 V. Therefore, the theoretical value of the slope of the plot is 0.02958 V.
The y-intercept of the plot corresponds to the value of E°cell. Since E°cell is the standard cell potential, it remains constant regardless of the concentration of the silver ions. Among the provided choices, the closest value to E°cell is 0.455 V. Hence, the theoretical value of the y-intercept is 0.455 V.
Learn more about Electrochemical cells
brainly.com/question/30375518
#SPJ11
what kind of bonds do alcohols form between individual molecules? a) hydrogen bonds b) ionic bonds c) oxygen bonds d) carbon bonds e) single bonds
Answers
Alcohols form hydrogen bonds between individual molecules. Hydrogen bonding occurs when a hydrogen atom bonded to an electronegative atom (such as oxygen or nitrogen) interacts with a lone pair of electrons on another electronegative atom.
In the case of alcohols, the oxygen atom is highly electronegative and forms a polar covalent bond with a hydrogen atom. This oxygen-hydrogen bond creates a partial positive charge on the hydrogen atom and a partial negative charge on the oxygen atom. These partial charges allow for hydrogen bonding to occur.
Hydrogen bonding is a strong intermolecular force that results in the formation of relatively stable and organized structures in liquids and solids. It plays a crucial role in determining many physical and chemical properties of alcohols, including their boiling points, solubility, and viscosity.
Therefore, the correct answer is a) hydrogen bonds. Alcohols, such as ethanol and methanol, form hydrogen bonds between individual molecules due to the presence of the oxygen-hydrogen bonds in their molecular structure.
Know more about Alcohols here:
https://brainly.com/question/29268872
#SPJ1
1. The un rie over the horizon. Frame of reference:
2. A bu move pat people tanding on the idewalk. Frame of reference:
3. A paenger on a train ee a ball roll down the aile. Frame of reference:
4. Two expre ubway train traveling at the ame peed on parallel track whiz pat paenger waiting on the platform of a local tation. Frame of reference:
5. A paenger on one of the ubway train look out the window and ee another train tanding till. Frame of reference:
6. A peron tanding near a railroad track ee a train pa by, then notice an airplane fly overhead in the ame direction a the train, but at a much fater peed. Frame of reference:
7. A paenger in the airplane look down and ee the train moving backward. Frame of reference:
Answers
1. The sun rise over the horizon. Frame of reference: Background
2. A bus moves past people standing on the sidewalk. Frame of reference: Moving
3. A passenger on a train sees a ball roll down the aisle. Frame of reference: Object
4. Two express subway trains traveling at the same speed on parallel track whiz past passenger waiting on the platform of a local station. Frame of reference: Moving
5. A passenger on one of the subway train looks out the window and see another train standing still. Frame of reference: Stationary
6. A person standing near a railroad track sees a train pass by, then notice an airplane fly overhead in the same direction as the train, but at a much faster peed. Frame of reference: Compare
7. A passenger in the airplane look down and sees the train moving backward. Frame of reference: Object
A frame of reference is a collection of coordinates that may be used to calculate the locations and velocities of objects inside that frame; various frames of reference move relative to one another. When you observe a ball roll down a street, you can know it's moving also because frame of reference is the roadway, whatever's on the side of the road, or the Earth. These are all frames of reference. All motion data will be linked to a frame of reference.
To learn more about Frame of references, here
https://brainly.com/question/1217098
#SPJ4
a student places iron metal over a flame, and begins to react with oxygen gas in the air. A red, powdery solid begins to form on the iron. What is the name of the red solid?
Answers
Answer:
Iron, as well as iron alloys, rusts because of a chemical reaction known as oxidation. When iron is exposed to moisture or oxygen, oxidation occurs. During this chemical reaction, iron is converted into iron oxide
A cinder block is sitting on a platform 20 m high. It weighs 79 N. The block has ____________ energy.
A. Kinetic
B. Potential
Answers
Answer:kinetic
Explanation:
Thank
Answer:
B.
Explanation:
The block has potential energy.