How did Moseley establish a more accurate periodic table?
Answers
Related Questions
How many grams of hydrogen chloride can be formed from 80.00 g of chlorine?
Answers
The balanced chemical equation for the reaction between chlorine and hydrogen to form hydrogen chloride is:
Cl2 + H2 → 2HCl
From the balanced chemical equation, 1 mole of chlorine produces 2 moles of hydrogen chloride. Therefore, the number of moles of hydrogen chloride produced from 2.26 mol of chlorine is:
Number of moles of HCl = 2 × Number of moles of Cl2
= 2 × 2.26 mol
= 4.52 mol
Finally, to determine the mass of hydrogen chloride produced, we need to multiply the number of moles of hydrogen chloride by its molar mass, which is 36.46 g/mol.
Mass of HCl = Number of moles of HCl × Molar mass of HCl
= 4.52 mol × 36.46 g/mol
= 164.9 g
80.00 g of chlorine can produce 164.9 g of hydrogen chloride.
To know more about chemical visit :-
https://brainly.com/question/28789639
#SPJ11
How can water be changed into ice
Answers
Answer:
- The molecules stick together and form a solid making ice
- By removing heat
which of the following options correctly defines resonance structures? multiple choice question. lewis structures for the same species that differ in the placement of electrons lewis structures that have the same molecular formula but a different placement of atoms lewis structures for the same species that have a different total number of electrons lewis structures that contain multiple bonds
Answers
The Lewis structures with many bonds in various places are the appropriate answer.
Resonance structures refer to Lewis structures for the same species that have multiple bonds in different locations. These structures have the same molecular formula and
the same placement of atoms, but differ in the distribution of electrons. This means that the atoms in the molecule can have different arrangements of double bonds or lone pairs of electrons, resulting in variations in the actual structure of the molecule.
Therefore, the correct option is lewis structures that contain multiple bonds in different locations.
To learn more about : Lewis
https://brainly.com/question/20300458
#SPJ11
When the equation 4. 78X^2-2. 14x-1. 60=0 is solved the two values of unknown x are ____ and _____
Answers
x has the values (2.14 + √(23.288)) / 9.56 and (2.14 - √(23.288)) / 9.56.
What Is a Quadratic Equation?Quadratic equations are the degree two, one-variable polynomial equations of the form f(x) = ax^2 + bx + c = 0 with a, b, c, and R R and a 0 being the variables. In its most basic form, it is a quadratic equation where "a" denotes the leading coefficient and "c" is the absolute term of f(x). The values of x that satisfy the equation (, ) are the quadratic equation's roots.
The name "quadratic equation" is for what?Intricate relationships exist between square and quadrangle (another name for rectangle) difficulties and quadratic equations.
The Latin term quadratus, which means square, is where the word "quadratic" originates.
The quadratic formula or factoring can be used to get the solution to the problem 4.78x^2-2.14x-1.60=0.
x = (-b (b^2 - 4ac)) / (2a) where a = 4.78, b = -2.14, and c = -1.60 is the quadratic formula.
Plugging in the values we get:
x = (-(-2.14) ± √((-2.14)^2 - 4(4.78)(-1.60)) ) / (2(4.78))
x = (2.14 ± √(4.6016 + 18.688)) / 9.56
x = (2.14 ± √(23.288)) / 9.56
So the two values of x are:
x = (2.14 + √(23.288)) / 9.56 and x = (2.14 - √(23.288)) / 9.56
To know more about Quadratic equations visit: brainly.com/question/30098550
#SPJ4
fructose draw structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol or enone below.
Answers
Carbonyl bonds are quite polar. Because oxygen is more electronegative than carbon, there is a partial positive charge on the carbon and a partial negative charge on the oxygen. The double bond between carbon and oxygen intensifies this charge separation.
Carbonyl carbon is an electrophile that combines with nucleophiles to form a tetrahedral species. It is the most basic reaction in carbonyl chemistry. You can now add an acid to a carbonyl to make it even HUNGRY for nucleophiles.
Turbo-charged nucleophiles include enamine, enolates, and enols. The alpha carbon atom is the nucleophilic atom.
Despite the fact that that carbon is a double bonded carbon with no lone pair, that position is motivated to donate electrons due to pi donation from the oxygen.
To know more about carbonyl electrophile visit
https://brainly.com/question/28203269
#SPJ4

A chemistry student weighs outof phosphoric acid, a triprotic acid, into avolumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid withsolution.
Calculate the volume ofsolution the student will need to add to reach the final equivalence point. Round your answer tosignificant digits.
Answers
To calculate the volume of solution needed to reach the equivalence point, we need to know the concentration of the phosphoric acid solution in the volumetric flask. Let's assume that the student weighed out 0.1 moles of phosphoric acid and dissolved it in a 250 mL volumetric flask, resulting in a concentration of 0.4 M (0.1 moles / 0.25 L).
Since phosphoric acid is a triprotic acid, it can donate up to three protons (H+ ions) in a reaction. To fully titrate the acid, we need to add three equivalents of a solution that can accept these protons. Let's assume the solution used for titration is sodium hydroxide (NaOH), which can accept one proton per molecule.
The balanced chemical equation for the reaction between phosphoric acid and sodium hydroxide is:
H3PO4 + 3 NaOH → Na3PO4 + 3 H2O
From the equation, we can see that for every mole of phosphoric acid, we need three moles of NaOH to reach the equivalence point.
Therefore, the number of moles of NaOH needed to titrate the 0.1 moles of phosphoric acid is:
0.1 moles H3PO4 x 3 moles NaOH / 1 mole H3PO4 = 0.3 moles NaOH
To calculate the volume of 0.3 M NaOH solution needed to provide 0.3 moles of NaOH, we can use the formula:
moles = concentration x volume
Rearranging the formula, we get:
volume = moles / concentration
Plugging in the values, we get:
volume = 0.3 moles / 0.3 M = 1 L
Therefore, the chemistry student will need to add 1 liter (or 1000 mL) of 0.3 M NaOH solution to the phosphoric acid solution in the volumetric flask to reach the equivalence point.
https://brainly.com/question/1434653
#SPJ11
. Which of the following is not a product of photosynthesis?
O A. Carbon dioxide
O B. Glucose
O C. Oxygen
Answers
Answer:
The answer would be a
Explanation:
That is what goes into the plant and oxygen IS an outcome
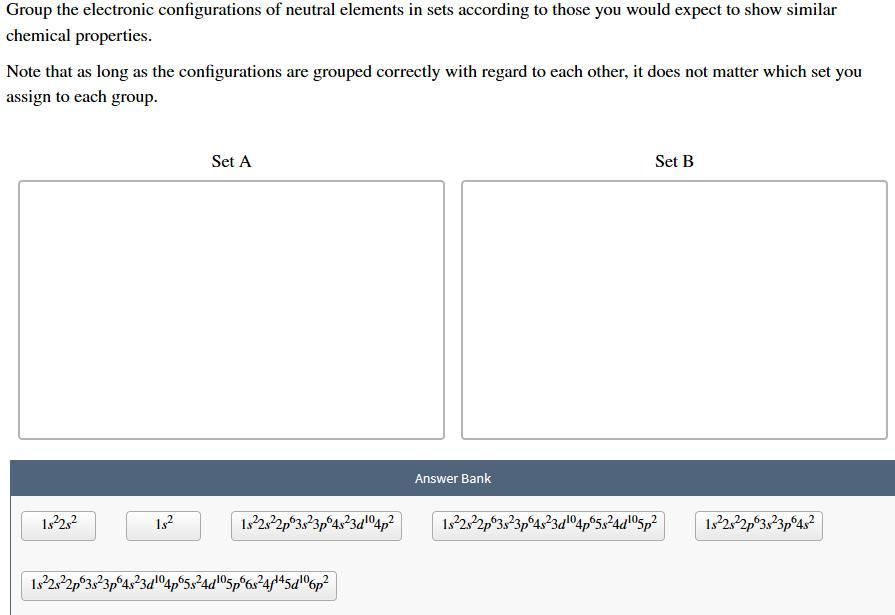
Group the electronic configurations of neutral elements in sets according to those you would expect to show similar chemical properties. Note that as long as the configurations are grouped correctly with regard to each other, it does not matter which set you assign to each group. Set A Set B Answer Bank 1:22:2 182 1922s22p03823p64323204p2 1322322p03823p 4323d"°4p65324d05p2 1922,22p3:23p6432 1.322s22p 3,23p64323 5p6s2445dº6p2
Answers
Set A elements behave similarly due to valence electrons in s and p orbitals, while Set B elements behave differently due to valence electrons in both s and d orbitals.
Set A:
1s²2s²
1s²
1s²2s²2p⁶3s²3p⁶4s²
Set B:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p²
The elements in Set A have valence electrons only in the s and p orbitals of their outermost energy level. This makes them similar in chemical behavior, as they tend to form covalent bonds by sharing electrons.
The elements in Set B have valence electrons in both the s and d orbitals of their outermost energy level, which gives them unique chemical properties, including the ability to form coordination complexes and exhibit variable oxidation states. Therefore, they are different in chemical behavior compared to the elements in Set A.
Learn more about neutral elements here: brainly.com/question/30433564
#SPJ4
Complete question is in the image attached below
Which of the following is a carbon sink?
A. Humans
B. Oceans
C. Burning fossil fuels
D. Livestock.
Answers
Answer: The ocean, soil and forests are the world's largest carbon sinks.
Answer:
B.Oceans
Explanation:
the answer is B. oceans
How many moles of aluminum are required to completely react with 107 mL of 6.00 M H2SO4 according to the balanced chemical reaction:
2 Al(s) + 3 H₂SO₄(aq) → Al₂(SO₄)₃(aq) + 3 H₂(g)
Answers
0.428 moles of aluminum are required to completely react with 107 mL of 6.00 M \(H2SO4\).
To solve this problem, we need to use the balanced chemical equation and stoichiometry to determine the number of moles of aluminum required to react completely with 107 mL of 6.00 M \(H2SO4\).
First, we need to determine the number of moles of \(H2SO4\)present in 107 mL of 6.00 M solution:
Molarity (M) = moles of solute / liters of solution
We can rearrange this equation to solve for moles of solute:
moles of solute = Molarity (M) x liters of solution
We have the volume of the solution in milliliters, so we need to convert it to liters:
107 mL = 0.107 L
Now we can calculate the moles of \(H2SO4\) present in 107 mL of 6.00 M solution:
moles of\(H2SO4\) = 6.00 M x 0.107 L = 0.642 moles
According to the balanced chemical equation, the stoichiometric ratio between aluminum and \(H2SO4\) is 2:3. This means that for every 2 moles of aluminum, 3 moles of \(H2SO4\) are required for a complete reaction.
So we can set up a proportion to find out how many moles of aluminum are required to react with 0.642 moles of\(H2SO4\):
2 moles Al / 3 moles\(H2SO4\) = x moles Al / 0.642 moles \(H2SO4\)
Cross-multiplying gives:
2 moles Al x 0.642 moles \(H2SO4\) = 3 moles \(H2SO4\) x x moles Al
Simplifying:
x = (2 moles Al x 0.642 moles\(H2SO4\)) / 3 moles \(H2SO4\) = 0.428 moles Al
To know more about aluminum refer to-
https://brainly.com/question/9496279
#SPJ11
How can the actions of people affect runoff and absorption in a positive way? How do they affect them in a negative way?
Answers
Answer:
pollution and waste every where like in are ouceans
Explanation:
People around us will affect us in positive or negative way. If someone is going to torture mentally or abuse by anything it will negatively impact on us. If some people support for our good deeds it will helps us positively.
What is influence from the surrounding?We are all social livings. We do influenced from our surrounding atmosphere, the people, place, climate everything. There will be positive things as well negative influences.
Some people behaves as anti social and they may ruin the life of others directly or indirectly. They may abuse others or being angry. These facts will make a person hate or the circumstances and will tend to runoff there.
Some other people always support for good beings and motivates others in their falls and hopeless conditions. From these kind of people, we can absorb the positive thoughts from positive people.
Good deeds and positive talks will make the surroundings also positives otherwise it will lead to negative thoughts and image that affect mental health.
To find more about positive thoughts, refer the link below:
https://brainly.com/question/4162137
#SPJ5
why do molecular compounds have low melting and boiling points and ionic compounds have high melting and boiling points?
Answers
There are two categories of chemical compounds. One is a covalent molecule, while the other is an ionic compound; covalent compounds are created when electrons are shared, but ionic compounds are created when all of the electrons are transferred.
What does a compound example mean?A material created by chemically joining two or more distinct components. Examples of compounds are table salt (NaCl), which is created from the elements sodium and chloride, and water (H2O), which is made from the components hydrogen and oxygen.
What is referred to as a compound?A compound is a material made up of two or more separate chemical components mixed together in a certain proportion. When the elements are combined, they react with one another and create chemical connections that are hard to dissolve.
To know more about Compound visit:
https://brainly.com/question/19458442
#SPJ1
structures consisting of four fused rings of carbon atoms with one or more polar hydroxyl groups attached to the ring structure are called
Answers
Steroids are the structures made up of four fused carbon atom rings with one or more polar hydroxyl groups affixed to the ring structure. The two main biological roles of steroids are as signaling molecules and as critical elements of cell membranes that affect membrane fluidity.
What do you mean by hydroxyl groups?
A functional group is one that contains the hydroxyl group (-OH). Alcohols are the compounds that are created when hydroxyl groups are the main functional group attached to carbon backbones. This group is located on the far right in the structural formula of the chemical molecule ethanol, which is a kind of alcohol.
Additional examples include propanol, methanol, and isopropyl alcohol.
To learn more about functional groups, click here: -
https://brainly.com/question/29263610
#SPJ4
A 25. 5ml aliquot of hcl (aq) of unknown concentration was titrated with 0. 113 m naoh (aq). It took 51. 2ml of the base to reach the endpoint of the titration. What is the concentration (m) of the acid?.
Answers
The concentration (m) of the acid is 0.227 M.
Titration is a commonplace laboratory technique of quantitative chemical analysis to determine the attention of an identified analyte. A reagent, termed the titrant or titrator, is ready as a trendy answer of recognized awareness and extent.
Calculation:-
C₁ = ?
V₁ = 25. 5ml = 0.0255 L
C₂ = 0. 113 M
V₂ = 51. 2ml = 0.0512 L
C₁V₁ = C₂V₂
C₁ = C₂V₂/V₁
= 0. 113 × 0.0512 L / 0.0255
= 0.227 M
The concentration of a substance is the quantity of solute found in a given amount of solution. Concentrations are normally expressed in terms of molarity, defined because of the variety of moles of solute in 1 L of answer.
The Concentration of an answer is a measure of the quantity of solute that has been dissolved in a given amount of solvent or answer. A concentrated answer is one that has a rather huge quantity of dissolved solute.
Learn more about concentration here:-https://brainly.com/question/26255204
#SPJ4
To form AgCl(s) from KCl(aq) and AgNO3(aq), the mass of KCl should be 10.00 g less than that of AgNO3. Find the mass of AgNO3.
Answers
Sodium nitrate & the insoluble substance silver chloride are produced when silver nitrate & sodium chloride combine.
Describe a compound?A substance made up of more than one element is called a compound. Table salt, water, and carbon dioxide are a few examples of compounds.
The two fundamental categories of compounds are. The way the particles in the molecule bond to one another defines who we are. They are referred to as "molecular" and "salt" compounds, respectively.
Describe silver?White metallic element with the highest electrical and thermal conductivity of any material which is sonorous, elastic, very malleable, able to undergo an elevated level of polish, and primarily monovalent in compounds. symbol See Table of Chemical Elements for Ag.Silver's scientific name is an acronym for the Latin.
To know more about compound visit :
https://brainly.com/question/13516179
#SPJ1
plz solve the question and send the answer
I will give u branist, follow u ,rate u 5 star and also give u like ,plz help me

Answers
Answer:
64g of \(\bold{CH_{3}OH}\dashrightarrow\)44.8L
vapour density of \(CH_{3}3OH=\frac{mass}{volume}\) of \(\bold{CH_{3}OH}\)
=64/44.8=10/7=1.43 g/l
Vapour density of \(\bold{CH_{3}OH}\)=1.43g/l
64g of \(\bold{CH_{3}OH => 44.8L }\)
vapour density of \(\small{\sf{CH_{3}3OH=\frac{mass}{volume} of } \bold{CH_{3}OH}}\)
=64/44.8=10/7=1.43 g/l
Vapour density of \(\bold{CH_{3}OH = 1.43 g/L}\)
please help thank you

Answers
Based on the Hund's Rule, the three electrons in a 2p orbital are arranged as shown in option C.
What is an orbital?The term orbital refers to a region in space where there is a high probability of finding the electron. We know that an electron in an atom could be defined by the use of four sets of quantum numbers which are;
The principal quantum numberThe orbital quantum numberThe spin quantum numberThe magnetic quantum numberHund's rule states that, electrons occur singly before pairing takes place. In this case, we can see that the only arrangement that obeys the Hund's Rule is option C.
Learn more about Hund's rule:https://brainly.com/question/12646067
#SPJ1
Does the sun reproduce
Answers
Answer: no
Explanation:
It aint got the dingdong and besides whos the mommyy??
the sun produces high frequency waves like gamma rays.
please help me asap 1.Draw in a paper by hand 1.Draw the molecular structure of the tetrapeptide Ser-Ala-Ala-Ser.The side chain of Ser is CH2-OH,and of Ala isCH3. 2.labelthe peptide bonds. 3.Write down the two sets of atoms you need to use to generate the dihedral angles of residue
Answers
To draw the molecular structure of the tetrapeptide Ser-Ala-Ala-Ser, follow these steps:
1. Start by drawing a line for the backbone chain, and attach the side chains of Ser and Ala accordingly.
2. Label the peptide bonds connecting each pair of amino acids.
3. Identify the two sets of atoms needed to generate the dihedral angles of each residue.
To draw the molecular structure of the tetrapeptide Ser-Ala-Ala-Ser, you can begin by drawing a straight line to represent the backbone chain. Next, attach the side chain of Ser, which consists of a CH2-OH group, to the first amino acid in the sequence. Then, attach the side chain of Ala, which is CH3, to the second amino acid. Repeat the process for the third amino acid, also an Ala, and finally, attach the side chain of Ser to the fourth amino acid.
To label the peptide bonds, mark the connections between adjacent amino acids along the backbone chain. Each peptide bond involves the amine group (NH) of one amino acid and the carboxyl group (COOH) of the neighboring amino acid.
To generate the dihedral angles of each residue, you would need to consider two sets of atoms. The first set includes the α-carbon (Cα), the carbonyl carbon (C), the amide nitrogen (N), and the α-hydrogen (Hα). The second set comprises the amide nitrogen (N), the α-carbon (Cα), the carbonyl carbon (C), and the carbonyl oxygen (O).
By considering the dihedral angles of the atoms in the peptide backbone, you can determine the conformational properties and structural characteristics of the tetrapeptide.
peptide structure and the principles of peptide bond formation to gain a deeper understanding of the molecular structure of tetrapeptides and the significance of dihedral angles in protein conformation.
Learn more about molecular structure
brainly.com/question/503958
#SPJ11
An incomplete diagram of meiosis is shown below. At the end of the process, how many chromosomes would be present in cell A?
Answers
In Cell A, there would be 8 chromosomes present at the end of the meiosis process.
What is the meiosis process?
Meiosis is a type of cell division that produces four daughter cells from one parent cell. It is a type of nuclear division that occurs in eukaryotic cells and is the process by which gametes (sex cells) are formed. The process of meiosis is composed of two separate divisions called meiosis I and meiosis II. During meiosis I, homologous chromosomes (which contain two copies of each gene) pair up, exchange genetic material, and then separate. This process is called crossing over and it creates genetic diversity. During meiosis II, the sister chromatids (copies of each chromosome) separate and move to opposite ends of the cell.
To learn more about meiosis process
https://brainly.com/question/15295733
#SPJ1
what is the process by which particles of fertilizer can cause foliar burn
Answers
Foliar burn is a plant condition caused by the application of fertilizer in excess. It appears as a leaf-tip or marginal burn, with the burning and dying of plant tissues, and the leaves will also display the formation of necrotic tissue and spots.
This happens because of the process by which particles of fertilizer can cause foliar burn.Foliar burn occurs when a fertilizer solution is applied to the plant’s foliage, and the solution stays on the leaves for too long. The particles of fertilizer can create an osmotic pressure difference across the leaf membrane, which leads to an imbalance of water between the leaf cells and the external environment.
This imbalance causes the plant cells to leak out, leading to cell death. As the plant cells die, the leaves start to turn brown and become brittle. In some cases, the leaves will fall off entirely.A long answer to your question can be: The process by which particles of fertilizer can cause foliar burn is the imbalanced water between the leaf cells and the external environment.
To know more about Foliar burn visit:-
https://brainly.com/question/31819209
#SPJ11
The chemical equation below represents one of the most important reactions for life on Earth. It describes how plants (and some microorganisms) combine water, sunlight, and carbon dioxide to produce the main source of food for most living organisms, glucose. A byproduct of this reaction is the oxygen we breathe. Is this reaction balanced? You must explain your reasoning for full credit!
PLS HELP, 30 Points!
I also need to know what I need to add to the equation to balance it!

Answers
The reaction is not balanced because the number of atoms of the elements on the reactant side isn't the same or equal to that on the product side.
What is Photosynthesis?This is referred to as the process in which green plants manufacture their food in the presence of sunlight and other compounds and is why they are regarded as primary producers in the ecosystem.
The balanced equation for photosynthesis is:
6CO₂ + 6H₂O + light --> C₆H₁₂O₆ + 6O₂
In this, we can see that the number of atoms of the elements of Carbon, hydrogen and oxygen are 6, 12 and 18 which is the same on the reactant and product side which is why the equation given in the question isn't balanced.
Read more about Photosynthesis here https://brainly.com/question/19160081
#SPJ1
the symbol for the metric unit used to measure mass is?
Answers
Answer: Kilograms (kg)
Explanation:
Answer:
(g)
Explanation:
(g) is the symbol of a gram which is the metric unit that measures mass.
Hope that helps!
what happend to that mass when the forest burns?

Answers
Answer:
When the forest burns we will lose a huge amount of oxygen and animals will lose their home.
Explanation:
The anion in the Finding Trends in Chemical Reactions Lab has little to no effect in the reactivity of the metal cations.
a) true
b) false
Answers
The anion in the Finding Trends in Chemical Reactions Lab has little to no effect in the reactivity of the metal cations" is false.
What is metal cations ?
A positively charged metal ion that has lost one or more electrons is known as a metal cation. In order to produce cations and develop a stable electronic configuration metals frequently lose electrons from their outermost shell.
Therefore, Students often examine the reactivity of various metal cations with various anions in the lab by monitoring the precipitate development. The choice of anion can influence the metal cation's solubility and reactivity which can have an impact on precipitate formation.
Learn more about metal cations here : brainly.com/question/30906831
#SPJ1
i’ll give Brainliest please answer also i know this isn’t chemistry but brainly doesn’t have science

Answers
the answer would be iron. divide mass by volume to get density.
Answer:
B. Iron
Explanation:
Correct
what is the bulk density of a dry soil sample with a
mass of 30 g that complely occupies a cylinder 6cm high and 4 cm in
diameter?
Answers
Answer:
397,570 g/m^3
Explanation:
The volume of the cylinder can be calculated using its height and diameter.
Mass of the soil sample (m) = 30 g
Height of the cylinder (h) = 6 cm
Diameter of the cylinder (d) = 4 cm
First, we need to calculate the radius (r) of the cylinder
Radius (r) = diameter / 2 = 4 cm / 2 = 2 cm = 0.02 m
Now, we can calculate the volume (V) of the cylinder
V = π * r^2 * h
V = 3.14159 * (0.02 m)^2 * 0.06 m
V = 7.5398 E-5 m^3
Calculate the bulk density (ρ) using this formula
ρ = m / V
ρ = 30 g / 7.5398 E-5 m^3
ρ = 397,887 g/m^3
What do symbols tell you about the conditions of the reactions shown to the right
Answers
They convey specific details about the reactants, products, physical states, and reaction conditions. Here's what symbols can tell you:
Reactants and products
Physical states
Catalysts
Temperature and pressure conditions
Symbols in chemical reactions provide important information about the conditions under which the reactions occur. They convey specific details about the reactants, products, physical states, and reaction conditions. Here's what symbols can tell you:
Reactants and products: Chemical formulas of reactants and products are represented by symbols, which indicate the identities of the substances involved in the reaction.
Physical states: Symbols such as (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solution indicate the physical state of the substances. This information helps in understanding the phase changes and solubility of the reactants and products.
Catalysts: Symbols like Pt, Ni, or enzymes indicate the presence of catalysts in the reaction. Catalysts facilitate the reaction without being consumed and are not included in the overall stoichiometry.
Temperature and pressure conditions: Symbols like ΔH (enthalpy change) and ΔS (entropy change) indicate temperature and energy changes associated with the reaction. Additional symbols may represent specific temperature or pressure conditions.
Overall, symbols in chemical reactions provide concise information about the substances involved, their physical states, and any special conditions or catalysts influencing the reaction. Understanding these symbols helps in interpreting and analyzing chemical equations.
For more question on products
https://brainly.com/question/30667391
#SPJ8
How many moles are in 79.85 g of Iron (III) oxide?
Answers
Answer:
For Fe, it's 55.845 g/mol. So 7.85 / 55.845 = 0.141 mol.
in which labeled portion of the curve would you use the heat of vaporization to calculate the heat absorbed?
Answers
Answer: To calculate the heat absorbed using the heat of vaporization, you would use the portion of the curve labeled "Evaporation."
What is Heat of Vaporization?Heat of vaporization is the energy required to transform a liquid into a vapor at a constant temperature, and it is expressed in joules per mole. Heat of vaporization is also known as enthalpy of vaporization, and it is a function of the substance's properties, temperature, and pressure.
The enthalpy of vaporization, like other thermodynamic properties, is often displayed as a function of temperature in a phase diagram, which shows the physical conditions (pressure, temperature, volume, etc.) at which different phases of a substance are stable. The temperature at which the vaporization process occurs is the boiling point.
The boiling point is the temperature at which the vapor pressure equals the external pressure, allowing bubbles of vapor to form within the liquid. During the process, heat is consumed to transform a liquid into a vapor, which is the heat of vaporization.
Learn more about Heat of Vaporization here:
https://brainly.com/question/30603212#
#SPJ11