How do you calculate radiation shielding?
Answers
"The protection of individuals against detrimental consequences of exposure to ionizing radiation and the methods of doing this" is how the International Atomic Energy Agency (IAEA) defines radiation protection, often known as radiological protection. Radiation from outside the body or radioactive contamination ingested via food or water may both induce exposure.
To calculate radiation shielding, follow these steps:
1. Identify the type of radiation: Determine whether it is alpha, beta, or gamma radiation, as each type requires different shielding materials.
2. Determine the energy of the radiation: The energy of the radiation, usually expressed in electron volts (eV), is essential to determine the appropriate shielding thickness.
3. Select a shielding material: Depending on the type of radiation, choose a suitable shielding material.
For example, alpha particles can be stopped by paper or clothing, beta particles require plastic or glass, and gamma radiation requires denser materials like lead or concrete.
4. Calculate the required thickness: Use the half-value layer (HVL) or tenth-value layer (TVL) concept to calculate the thickness needed for the shielding material. The HVL is the thickness of a material that reduces the radiation intensity by half, while the TVLreduces the intensity to one-tenth.
5. Apply the appropriate shielding: Based on your calculations, apply the necessary thickness of the chosen material to effectively shield against the radiation.
In summary, to calculate radiation shielding, you need to identify the type of radiation, determine its energy, select an appropriate shielding material, calculate the required thickness, and apply the shielding.
To know more about the radiation shielding https://brainly.com/question/3981055
#SPJ11
Related Questions
Calculate the number of moles in 3.440 x 10^35 formula units of RbF. (Molar mass of RbF is 104.47g/mol).
Answers
Fluorine (f), chlorine (cl), and bromine (br) can all react easily to form compounds with sodium (na). Which other element could most likely react to form a compound with sodium?.
Answers
Iodine is the most likely candidate since, first of all, it is included in the list of halogens together with all of the other elements.
What is an iodine used for?Iodine is used orally to prevent and cure iodine deficiency and also its effects, such as goiter but some thyroid problems. The US FDA has also approved potassium iodide, a particular kind of iodine, to protect thyroid damage following a radioactive mishap.
What are the risks associated with iodine?Inflammation of the thyroid gland and thyroid cancer can also result from high iodine consumption. A very high amount of iodine (several grams, for instance) can result in a coma, fever, stomach pain, nausea, vomiting, diarrhea, and burning in the mouth, throat, and stomach.
To know more about Iodine visit:
https://brainly.com/question/16867213
#SPJ1
which electrophile is used to make acetophenone from benzene?
Answers
The electrophile that is used to make the acetophenone from the benzene is the CH₃CO⁺.
The Acetophenone is the carbonyl compound in which the the group called as the ketone group is attached directly to the benzene ring. The catalyst that is used in the production of the acetophenone is the anhydrous aluminum chloride. This type of the reaction is a type of the electrophilic substitution reaction. The Electrophiles are the electron-deficient species and that are attracted to the electron - rich center. The electrophiles will accepts the electron pair.
The electrophile is CH₃CO⁺ that is used in the production of the acetophenone from benzene.
To learn more about electrophile here
https://brainly.com/question/30026567
#SPJ4
Metric prefixes 9th grade level
Answers
Answer:
A metric prefix is a unit prefix that precedes a basic unit of measure to indicate a multiple or submultiple of the unit. All metric prefixes used today are decadic. Each prefix has a unique symbol that is prepended to any unit symbol.name two examples which are not considered as matter
Answers
Answer:
Light and Sound can be considered as non-matter.
Explanation:
Various forms of Energy like Heat, Sound, Light can be considered as non-matter
which of the following correctly pairs a greenhouse gas with its primary anthropogenic source? responses methane and vehicular emissions methane and vehicular emissions nitrous oxide and agricultural practices nitrous oxide and agricultural practices chlorofluorocarbons and fossil fuel combustion chlorofluorocarbons and fossil fuel combustion carbon dioxide and municipal solid waste from homes
Answers
The correct pairing of a greenhouse gas with its primary anthropogenic source is: Methane and vehicular emissions.
The Greenhouse Effect is a naturally occurring process that is necessary for the maintenance of the planet's temperature. The sun's energy travels to Earth in the form of light. The Earth reflects some of this energy back to space while absorbing the remainder, which warms the planet.
Methane is a greenhouse gas that is released by several human activities, including fossil fuel production and use, landfills, agriculture, and livestock farming. Methane, like carbon dioxide, contributes to global warming by trapping heat in the Earth's atmosphere.
Vehicular emissions are the gases and particulate matter emitted by vehicles. They include carbon monoxide, nitrogen oxides, particulate matter, and other pollutants that are hazardous to human health and the environment.
Know more about greenhouse effect:
https://brainly.com/question/31595505
#SPJ12
chemical reaction that particulate matter undergoes that causes the problem
Answers
Particulate matter is a complicated combination of solid particles and liquid droplets in the air.
Industrial activities, transportation, and fossil fuel burning produce it. Composition, size, and other variables affect particulate matter's chemical reactions.
Particulate particles may react chemically in the atmosphere, including:
Oxidation: Particulate matter reacts with ambient gases like O2 and NOx to generate oxidised particles. Sulphate particles may arise from power plant and other SO2 emissions.
Nucleation: Atmospheric gases generate new particles. H2SO4 and NH3 gases may generate new particles.
Coagulation: Small particles mix to generate bigger particles. When microscopic particles hit and cling together, they generate bigger particles that settle more readily.
Photochemical reactions: Particulate matter exposed to sunlight undergoes photochemical reactions, forming new particles and changing their chemical makeup.
Learn more about Particulate matter, here:
https://brainly.com/question/15230454
#SPJ1
? Match the states of matter to their properties. Drag the items on the left to the correct location on the right. solids Indefinite shape, but definite volume liquids indefinite shape and indefinite volume M gases definite shape and definite volume lowest density particles glide past each other highest density Done Try hear
Answers
Answer:
Solids: definite shape and definite volume (highest density)
Liquid: indefinite shape and definite volume (glide past each other)
Gas: indefinite shape and indefinite volume (lowest density)
Explanation:
look at the answer
This is a reaction going on in your muscle cells right this very minute: what is the equilibrium constant for the un-catalyzed reaction?
Answers
The equilibrium constant for the un-catalyzed reaction is 0.9
The enzyme triose phosphate isomerase catalyzes the reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics.
Typical cellular concentrations:
triose phosphate isomerase = 0.1 nM
dihydroxyacetone phosphate = 5 µM
glyceraldehyde-3-phosphate = 2 µM
So, equillibrium constant is
K = \(\frac{[P]}{[S]}\)
K = \(\frac{ glyceraldehyde-3-phosphate}{dihydroxyacetone phosphate}\)
Therefore, The equilibrium constant for the un-catalyzed reaction is 0.9
Learn more about equillibrium here;
https://brainly.com/question/25657597
#SPJ4
PLEASE HELP!
What is the composition of the sigma bonds in methane i.e. what atomic orbits comprise the molecular orbit? Hint: they are all the same, so there is only one kind sigma bond here.
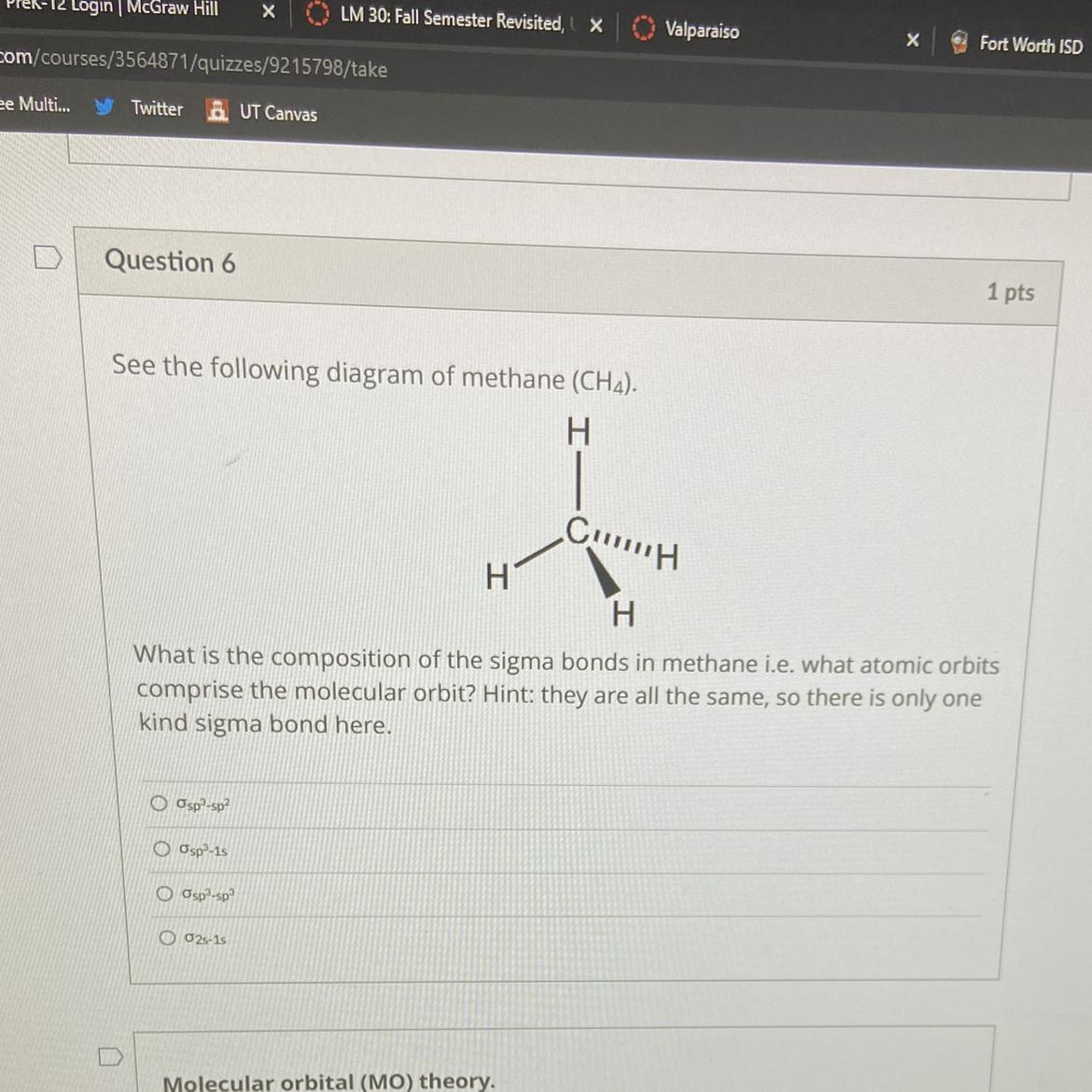
Answers
The 1s-orbital from the a hydrogen to either a sigma bond forms a link with one of the four sp3 hybrid orbitals of carbon.
What has a methane destructor?Methane is naturally eliminated by a variety of biological and chemical processes, including the reaction of methane-eating microorganisms (methanotrophs) on soil and water with air hydroxyl [OH] and chlorine.
How is methane dangerous to people?The quantity of oxygen absorbed from the air might be decreased by high methane levels. This may include headaches, facial flushes, slurred speech, visual issues, memory loss, changes in mood, and vision abnormalities. In severe situations, respiration and heart rate fluctuations, balance issues, numbness, and unconsciousness may occur.
To know more about methane visit:
https://brainly.com/question/2127750
#SPJ1
Which is a pure substance?
A) mixture
B) solution
C) element
Answers
when does a double replacement reaction occur
Answers
Answer:
u should call me justin bc I was just in your mom
Answer:
he periodic table or an activity series can help predict whether single-replacement reactions occur. A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. A precipitation reaction is a double-replacement reaction in which one
Explanation:
Molecules or ions that can alternately behave as either a Bronsted-Lowry acid or base are called a. polyanions b. hydronium ions c. conjugate acids or bases d. amphiprotic
Answers
Molecules or ions that can alternately behave as either a Bronsted-Lowry acid or base are called d. amphiprotic.
Amphiprotic species are capable of donating or accepting a proton (H+) and can exhibit both acidic and basic properties. The term "amphiprotic" is used in the context of the Bronsted-Lowry acid-base theory, which defines acids as proton donors and bases as proton acceptors.
Let's consider the other options to further clarify:
a. Polyanions: Polyanions are negatively charged ions composed of multiple atoms. They do not necessarily exhibit amphiprotic behavior unless they can donate or accept protons in a reversible manner.
b. Hydronium ions: Hydronium ions (H3O+) are formed when water molecules combine with an additional proton (H+). They act as Bronsted-Lowry acids by donating a proton and do not exhibit reversible behavior as a base.
c. Conjugate acids or bases: Conjugate acids or bases refer to species that are related to each other through the gain or loss of a proton. In the Bronsted-Lowry acid-base theory, a conjugate acid is formed when a base accepts a proton, and a conjugate base is formed when an acid donates a proton. While conjugate acids or bases are involved in acid-base reactions, they do not necessarily possess the ability to behave as both an acid and a base simultaneously.
Therefore, the correct answer is d. amphiprotic, as it specifically refers to molecules or ions that can alternately behave as either a Bronsted-Lowry acid or base, demonstrating both acidic and basic properties depending on the reaction and conditions.
To know more about conjugate acids visit:
brainly.com/question/16428518
#SPJ11
Nicolaas' model demonstrates that and are primarily responsible for the movement of water on earth
Answers
Nicolaas' model is a scientific model that explains the movement of water on Earth. According to the model, the two primary factors responsible for the movement of water on Earth are evaporation and precipitation.
Evaporation occurs when water changes from a liquid to a gas state due to heat from the sun. This process results in the formation of water vapor that rises into the atmosphere. Precipitation occurs when water vapor condenses in the atmosphere and falls back to the surface as rain, snow, or hail. These two processes play a critical role in the water cycle, which is essential for the survival of life on Earth. Therefore, Nicolaas' model highlights the significance of evaporation and precipitation in the movement of water on Earth.
Learn more about Nicolaas' model at https://brainly.com/question/15923461
#SPJ11
how would your calculations of the concentration of [fescn]2 been affected if the cuvette you used had a 1.5 cm path length rather than the 1.0 cm value you were told to use?
Answers
The increased distance across the cell will result in an increase absorbance reading.
The concentration of \([Fescn]_2\) would be affected if the cuvette had a 1.5 cm path length rather than the 1.0 cm value used.Since the absorbance of a sample is proportional to the concentration of a sample (as described by the Beer-Lambert law), increasing the path length of the cuvette would result in a decrease in absorbance. This means that the concentration of the sample would be lower than if the 1 cm path length was used. In other words, the concentration of \([Fescn]_2\)would be lower if the cuvette had a 1.5 cm path length than if it had a 1.0 cm path length.
learn more about cuvette Refer:brainly.com/question/29385690
#SPJ1
An alkali is able to “cancel” out an acid. What is the other word for “cancelling out the acid”?, Ill mark brainliest if your answer is correct ;))
a) neutralise
b) overcome
c) nullify
d) become acidic
Answers
Answer:
neutralise
Explanation:
There is a special name given to the reaction between an acid and a base to form salt and water and water only. That name is 'neutralization'. Hence to 'neutralize' an acid connotes the idea of 'cancelling' the effect of an acid.
An acid has a number of deleterious effects on surfaces, the most prominent of which is corrosion of the surface due to oxidation. If a surface is accidentally exposed to an acid, the effect of that acid on the surface can be counteracted by the immediate application of a base to 'neutralize' the acid. Neutralization in this sense is akin to 'cancelling' out the acid.
The examples are endless. Another common example is the neutralization of an acidic soil by a base in order to 'cancel' the acidity of the soil and make the soil fertile for plant growth.
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
How does the classroom temperature affect students’ performance in a test hypothesis ?
Answers
Arrange Cu, Ca, Na, Zn and Au on the basis of their decreasing reactivity.
Answers
Answer:
1. Sodium (Na) is the most reactive due to its lower number of shells which enables the protons in it's nucleus to attract more electrons.
2. Calcium (Ca) is next as it is a group 2 element and they can be quite reactive.
3. Zinc (Zn) is the third reactive as transition metals are not very reactive.
4. Copper (Cu) is less reactive than Zinc because it is closer to the center of the periodic table and therefore has more stability.
5. Gold (Au) is the least reactive.
Acetone, a highly volatile liquid, is placed in a closed container. The amount of liquid decreases, but eventually becomes stable. At that time... O the rate of evaporation is less than the rate of condensation O the rates of evaporation and condensation cannot be compared without more information O the rate of evaporation is greater than the rate of condensation the kinetic energy of each gaseous molecule is equal to the kinetic energy of each liquid molecule O the rate of evaporation is equal to the rate of condensation
Answers
Acetone, a highly volatile liquid, is placed in a closed container. The amount of liquid decreases, but eventually becomes stable. At that time the rate of evaporation is equal to the rate of condensation. The correct answer is option d.
At the time when the amount of acetone in the closed container becomes stable, the rate of evaporation is equal to the rate of condensation. This is because, in a closed system, the rate of evaporation and condensation will eventually reach a balance, where the number of molecules leaving the liquid phase is equal to the number of molecules returning to the liquid phase. At this point, the net change in the amount of acetone in the container will be zero, and the liquid will remain stable.
It's important to note that the rates of evaporation and condensation are influenced by several factors, such as temperature, pressure, and the surface area of the liquid exposed to the air. However, in a closed system, these factors will eventually reach a balance, leading to a stable state where the rate of evaporation is equal to the rate of condensation.
To know more about condensation refer to-
brainly.com/question/15563071#
#SPJ11
Complete question
Acetone, a highly volatile liquid, is placed in a closed container. The amount of liquid decreases, but eventually becomes stable. At that time
a. the rate of evaporation is less than the rate of condensation
b. the rates of evaporation and condensation cannot be compared without more information
c. the rate of evaporation is greater than the rate of condensation the kinetic energy of each gaseous molecule is equal to the kinetic energy of each liquid molecule
d. the rate of evaporation is equal to the rate of condensation
3.4 x 10^23 atoms of potassium to grams
Answers
To solve this problem, we must take into account Avogadro's number. Avogadro's number tells us that in one mole of any substance there are 6.022x10^23 atoms. Applying this relationship we have that the moles of potassium (K) are:
\(\begin{gathered} molK=givenatomsK\times\frac{1molK}{6.022\times10^{23}atoms} \\ \end{gathered}\)\(\begin{gathered} molK=3.4\times10^{23}atomsK\times\frac{1molK}{6.022\times10^{23}atoms} \\ molK=0.6molK \end{gathered}\)Now, to go from moles to grams, we must multiply the moles by the molar mass of potassium. The molar mass of potassium is 39.1g/mol. So, the grams of potassium will be:
\(gK=givenmolK\times\frac{MolarMass,gK}{1molK}\)\(\begin{gathered} gK=0.6molK\times\frac{39.1gK}{1molK} \\ gK=22.1gK \end{gathered}\)Answer: In 3.4x10^23 atoms of potassium there are 22.1 grams
147.0 g piece of metal was heated to 155 C and added to a calorimeter containing 75.0 grams of water. The temperature of the water in the calorimeter was 23.5 C. After the metal was added, the temperature rose to 33.5 C. What is the specific heat of the metal?
Answers
Based on the properties in Table 1, which example describes the best use of nitinol in a manufactured product?
Answers
Answer: D- making flexible eyeglass frames
Explanation: my powerful mind
What is the planet's albedo? group of answer choices its ability to reflect light its ability to produce carbon dioxide its ability to absorb light its ability to product stratospheric ozone
Answers
The planet's albedo has ability to reflect light.
The planetary albedo would be the percentage of incoming solar radiation that Earth scatters back into space. The processes that control the quantity, distribution, and fluctuation of this reflected energy are crucial to the Earth's energy balance and have a significant impact on both climate including climate change.
Temperatures rise as a result of carbon dioxide, prolonging the growing season as well as raising the humidity. Each of these elements has stimulated some further plant growth. But hotter weather also stresses plants. Plants require more water to live in an extended, warmer growing season.
Therefore, the planet's albedo has ability to reflect light.
To know more about planet's albedo
https://brainly.com/question/7138899
#SPJ4
a diatomic element with a high first ionization energy would most likely be a
Answers
A diatomic element with a high first ionization energy would most likely be a nonmetal.
Diatomic elements are elements that naturally form molecules containing two atoms of the same element. Examples include hydrogen (H₂), oxygen (O₂), and nitrogen (N₂). First ionization energy is the energy required to remove one outermost electron from an atom in its gaseous state. Nonmetals, which are found on the right side of the periodic table, generally have higher first ionization energies compared to metals. This is because they have a stronger attraction to their outermost electrons, making it more difficult to remove them. Hence, a diatomic element with a high first ionization energy would most likely be a nonmetal.
To learn more about ionization energy https://brainly.com/question/20658080
Pls help me I don’t know how to do this

Answers
Explanation:
We have a 63.9 g sample of calcium hydroxide. First we have to convert those grams into moles. To do that we have to use the molar mass of calcium hydroxide.
Calcium hydroxide = Ca(OH)₂
molar mass of Ca = 40.08 g/mol
molar mass of O = 16.00 g/mol
molar mass of H = 1.01 g/mol
molar mass of Ca(OH)₂ = 1 * 40.08 g/mol + 2 * 16.00 g/mol + 2 * 1.01 g/mol
molar mass of Ca(OH)₂ = 74.10 g/mol
mass of Ca(OH)₂ = 63.9 g
moles of Ca(OH)₂ = 63.9 g /(74.10 g/mol)
moles of Ca(OH)₂ = 0.862 moles
In 1 molecule of Ca we have 2 atoms of O. So in 1 mol of Ca(OH)₂ we will have 2 moles of O atoms.
1 mol of Ca(OH)₂ = 2 moles of O atoms
moles of O atoms = 0.862 moles of Ca(OH)₂ * 2 moles of O /1 mol of Ca(OH)₂
moles of O atoms = 1.724 moles
One mol is similar to a dozen. When we say that we need a dozen eggs we know that we need 12 eggs. If we want a mol of eggs, we want 6.022*10^23 eggs. So one mol of something is 6.022 * 10^23 of that.
1 mol of O atoms = 6.022 * 10^23 atoms
n° of O atoms = 1.724 moles * 6.022 * 10^23 atoms/1 mol
n° of O atoms = 1.04 * 10^24 atoms
Answer: In a 63.9 g sample of Ca(OH)₂ we have 1.04 *10^24 atoms of oxygen.
4 (c) Aluminium is produced by electrolysis of a molten mixture of aluminium oxide and cryolite. This is shown in Figure 3.
Name a gas produced at the positive electrode. Gas forms at the positive electrode Aluminium forms at the negative electrode [1 mark]
Answers
How many molecules of SF6
are in 25.0 g SF6?
[ ? ]×10⁰²] molecules SF6
Answers
SF₆
Molar mass: 146.06 g/mol
mole = 25 : 146.06 = 0.171
molecules:
=0.171 x 6.02 x 10²³
=1.03 x 10²³
1. How does our Sun produce energy for all of the heat and light that we get?
2. What did you learn about fusion from these resources?
Please help me in your own words
Answers
1. the Earth's core products light to me. I like it. cuz it give.me light.
2. I learned that the fusion from these resources.
Find w, xx, yy and zz such that the following chemical reaction
is balanced.
32+xH2→y(H)2+zH3
Answers
In order to balance the chemical equation 32 + xH2 → y(H)2 + zH3, we need 32 moles of hydrogen gas (H2), x = 16 moles of H2, y = 32 moles of H, and z = 16 moles of H3.
To balance a chemical equation, we need to ensure that the number of atoms on both sides of the equation is equal. In this case, we have 32 hydrogen atoms (H) on the left side, represented by xH2, and we need to determine the values of x, y, and z to balance the equation.
On the right side, we have y(H)2, which means we have 2y hydrogen atoms. Similarly, we have zH3, which represents 3z hydrogen atoms.
To balance the equation, we need to find values for x, y, and z that satisfy the condition. Since we have 32 hydrogen atoms on the left side, we can set up the equation:
2y + 3z = 32
To simplify the equation, we can divide both sides by the greatest common divisor of 2 and 3, which is 1. This gives us:
2y + 3z = 32
To find a solution for this equation, we can try different values for y and z that satisfy the equation. After some trial and error, we find that y = 32 and z = 16 satisfy the equation.
Therefore, the balanced chemical equation is:
32 + 16H2 → 32(H)2 + 16H3
Learn more about balancing a chemical equation :
https://brainly.com/question/14072552
#SPJ11