Answers
Explanation: C) the air temp. at the top is lower
Related Questions
A sample of trifluoroacetic acid, C2HF3O2, contains 47.3 g of oxygen. Calculate the mass of the trifluoroacetic acid sample.
Answers
Based on the percentage mass of oxygen in trifluoroacetic acid, the mass of the trifluoroacetic acid sample is 168.5 grams.
What is the percentage mass of oxygen in trifluoroacetic acid?The percentage mass of oxygen in trifluoroacetic acid is determined as follows:
The molar mass of trifluoroacetic acid = 12 * 2 + 1 + 19 * 3 + 16 * 2
The molar mass of trifluoroacetic acid = 114 g
The percentage mass of oxygen in trifluoroacetic acid = (16 * 2)/114 * 100%
The percentage mass of oxygen in trifluoroacetic acid = 28.07%
The mass of the trifluoroacetic acid sample will be 47.3/ 28.07% = 168.5 grams
Learn more about percentage mass at: https://brainly.com/question/26150306
#SPJ1
Which fundamental force acts over the shortest distance?
O A. Strong nuclear force
B. Electrostatic force
O C. Weak nuclear force
D. Gravitational force
Answers
It is much weaker than the electric and the strong forces (but still much stronger than gravity at short distances), acts over an extremely tiny distance, and is the cause for beta decay in atoms. So the correct answer is C
Answer:
C. Weak nuclear force
Explanation:
The weak force is responsible for radioactive decay and neutrino interactions. It has a very short range and. As its name indicates, it is very weak. The weak force causes Beta-decay ie. the conversion of a neutron into a proton, an electron and an antineutrino.
Help Anyone! What would be the correct answer to this question?

Answers
Underline the correct answer 1.In which of the following substances are the particles arranged in a regular pattern? A Water B Salt solution C Ice D Water vapour
Answers
Answer:
A). Water
PLEASE GIVE BRAINLIEST THANK YOU!!!
Answer:
water
Explanation:
1. Related to the number of particles in a gram of
substance and to possible motions of the
particles (2 words)
Answers
Answer:
Avogadro's number or Avogardro’s constant
Explanation:
I’m pretty sure this is correct if it’s not I’m sorry lol.
9. What tool do you use to measure the mass of an object?
Answers
Answer:
triple beam balance
Explanation:
The answer is:
Balance
Mass is the amount of matter in an object. Scientists often measure mass with a balance. A type of balance called a triple beam balance is pictured in Figure below.
Hope this helps!
An iceberg has a volume of 0.1642 ML. What is the mass of the ice(in kg) composing the iceberg( at 0o C)? The density of ice at 0o C is 0.917g/cm^3
Answers
Answer:
1.5x10¯⁴Kg
Explanation:
Data obtained from the question include the following:
Volume = 0.1642mL = 0.1642cm³
Density = 0.917g/cm³
Mass =.?
The Density of a substance is simply defined as the mass per unit volume of the substance. Mathematically, it is represented as:
Density = Mass /volume
With the above formula, we can calculate the mass of the ice as follow:
0.917 = Mass / 0.1642
Cross multiply
Mass = 0.917 x 0.1642
Mass = 0.151g
Finally, we shall convert 0.1506g to kg. This is illustrated below:
1000g = 1k
Therefore, 0.151g = 0.151/1000 = 1.5x10¯⁴Kg
422000 scientific notation form:
Answers
if the temperature is 345 K, what is the temperature in C°
Answers
Answer:
71.85 Celsius (°C)
Explanation:
If the temperature is 345 K then the temperature is 71.850 Celsius.
Definition: A one or two letter abbreviation for a chemical element.
Example: Cu is copper.
Answers
Answer:
chemical symbol
Explanation:
What is the difference between a polymer and a plastic?
Answers
Rather than presenting a marked difference between plastics and polymers, it should be emphasized that polymers are a group of compounds that encompass large molecules formed by the union of covalent bonds. Within the polymers there are some subgroups, these subgroups are classified according to the properties that the polymer has and its origin. They can come from natural or synthetic sources.
Plastics are a subgroup of synthetic polymers that possess temperature-sensitive properties, that is, they deform at a certain temperature, so their molding is done with heat.
Complexes containing metals with d10 electron configurations are typically colorless because ________. Complexes containing metals with d10 electron configurations are typically colorless because ________. d electrons must be emitted by the complex in order for it to appear colored there are no d electrons to form bonds to ligands a complex must be charged to be colored there is no d electron that can be promoted via the absorption of visible light the empty d orbitals absorb all of the visible wavelengths
Answers
Answer:
there is no d electron that can be promoted via the absorption of visible light
Explanation:
One of the properties of transition elements is the possession of incompletely filled d orbitals. This property accounts for their unique colours.
The colours of transition metal compounds stem from d-d transition of electrons due to the presence of vacant d orbitals of appropriate energy to which electrons could be promoted.
For elements whose atoms have a d10 configuration, such vacant orbitals does not exist hence their compounds are not colored.
Sometimes, the colour of transition metal compounds stem from ligand to metal charge transfer(LMCT) for instance in KMnO4.
How many years passed between the first man on the Moon and the first US woman in space?
Answers
It took 14 years between the first man on the Moon and the first US woman in space.
What is space exploration?The use of astronomy and space technology to explore outer space is known as space exploration. While astronomers use telescopes to explore space, physical exploration is carried out by both uncrewed robotic space probes and human spaceflight.
Understanding gravity, the magnetosphere, the atmosphere, fluid dynamics, and the geological evolution of other planets, for example, has come from studying the solar system.
Learn more about space exploration here:
https://brainly.com/question/27325845
#SPJ1
This chart shows global energy usage for the year 2005. Solar, 0.5% Hydroelectric, 3% Wind, 0.3% Biomass Geothermal, 0.2% Nuclear Oil 379 Natural gas 23% Need an extra pair of e Get writing feedback fri real tutor Submit a review Coal Use the chart to answer the following questions. (8 points) A. What total percent of energy came from fuels that emitted greenhouse gases?
Answers
Approximately 60.9% of the total energy in 2005 came from fuels that emitted greenhouse gases. This signifies a significant contribution to global greenhouse gas emissions and highlights the importance of transitioning to cleaner and more sustainable energy sources to mitigate climate change impacts.
To determine the total percent of energy that came from fuels emitting greenhouse gases, we need to consider the energy sources listed in the chart that are known to produce greenhouse gas emissions. In this case, those would be oil, natural gas, and coal.
From the chart, we see that the percentages for these three energy sources are:
Oil: 37.9%
Natural gas: 23%
Coal: Not specified
Although the percentage for coal is not mentioned in the given information, it is a known fact that coal combustion releases greenhouse gases, including carbon dioxide (CO2). Therefore, we can assume that coal is among the fuels emitting greenhouse gases.
Adding up the percentages for oil and natural gas, we have:
37.9% (oil) + 23% (natural gas) = 60.9%
Therefore, approximately 60.9% of the total energy in 2005 came from fuels that emitted greenhouse gases. This signifies a significant contribution to global greenhouse gas emissions and highlights the importance of transitioning to cleaner and more sustainable energy sources to mitigate climate change impacts.
For more question on energy
https://brainly.com/question/30745996
#SPJ11
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
Select the best answer for the question.
11. Iron(II) is available to bond with chloride ion. How many of each type of ion will bond to form an ionic compound?
O A. 2 iron(II), 3 chloride
OB. 1 iron(II), 2 chloride
C. 2 iron(II), 1 chloride
D. 3 iron(II), 1 chloride
Answers
The correct answer is A. 2 iron(II), 3 chloride, that is 2 iron(II) ions and 2 chloride ions will bond to form an ionic compound
In an ionic compound, the total positive charge of the cations should balance the total negative charge of the anions. Iron(II) is a cation with a charge of +2 (Fe2+), while chloride is an anion with a charge of -1 (Cl-).
We need to balance the charges and hence need to determine the least common multiple (LCM) of the charges. The LCM of +2 and -1 is 2. Therefore, we need two chloride ions (2 x -1 = -2) to balance the charge of one iron(II) ion (+2).
Hence, for each ionic compound formed, we would need 2 iron(II) ions and 2 chloride ions to achieve charge neutrality. Therefore, the correct answer is A. 2 iron(II), 3 chloride.
for more questions on compound
https://brainly.com/question/29108029
#SPJ11
Identify which subatomic particles match each of these descriptions. One of the descriptions describes two particles. Make sure to include both particles it describes. Description Particle(s) have a relative charge of +1 have a relative charge of -1 have no charge located in the nucleus of an atom have a much lower mass than the other two types of particles Answer Bank protons electrons neutrons
Answers
Explanation:
protons have a relative charge of +1, they are located in the nucleus and the carry a positive charge
the electrons are negatively charged and have a charge of -1 . They are found orbiting on the shells .the electrons have a negligible mass of 1 / 1840
the neutrons have no charge they are located in the nucleus of an atom .
The first part of a balanced chemical reaction is shown 6CO2+6H2O.in order for this equation to be balanced how many oxygen atoms must be present in the products
Answers
We have the number of molecules of each reagent. The number before the molecule indicates the number present, the subindex after each atom tells us how many are inside the molecule and reflects the type of compound we have:
\(\begin{gathered} 6CO_2\text{ means that we have 6 mol of CO}_2 \\ \text{For each atom we will have: } \\ C\colon6\text{ x 1 =6} \\ O\colon\text{ 6 x 2 = 12} \end{gathered}\)We do the same for another molecule:
\(\begin{gathered} 6H_2O\text{ means that we have 6 mol of H}_2O \\ \text{For each atom we will have:} \\ H\colon\text{ 6 x 2 = 12} \\ O\colon\text{ 6 x 1 = 6} \end{gathered}\)If we add up all the atoms we will have:
C: 6
O: 12 + 6 = 18
H: 12
So, we will need 18 oxygen atoms (O) must be present in the products.
What is the anion of the ionic compound HC2H3O2?
Answers
Answer:
Can u write in a paper and post again unable to understand
Which symbol represents salt?
Question 10 options:
C2H2
O2
C6H12O6
KBr
Answers
Answer:none
Explanation:none of these represent salt,i know that it's NaCl
How to Balance __MgF2 + __Li2CO3 + __ LiF
Answers
What amount of heat is required to vaporize 143.45 g of ethanol (C₂H₅OH)? (∆Hvap = 43.3 kJ/mol)
Answers
Answer:
135 KJ.
Explanation:
The following data were obtained from the question:
Mass of ethanol, C₂H₅OH = 143.45 g
Heat of vaporisation, ∆Hvap = 43.3 kJ/mol
Heat required (Q) =.?
Next, we shall determine the number of mole in 143.45 g of ethanol (C₂H₅OH). This is can be obtained as follow:
Mass of ethanol, C₂H₅OH = 143.45 g
Molar mass of ethanol, C₂H₅OH = (12×2) + (1×5) + 16 + 1
= 24 + 5 + 16 + 1
= 46 g/mol
Mole of ethanol, C₂H₅OH =?
Mole = mass /Molar mass
Mole of ethanol, C₂H₅OH = 143.45/46
Mole of ethanol, C₂H₅OH = 3.118 moles
Finally, we shall determine the heat required to vaporize the ethanol, C₂H₅OH as follow:
Heat of vaporisation, ∆Hvap = 43.3 kJ/mol
Mole of ethanol, C₂H₅OH (n) = 3.118 moles
Heat required (Q) =.?
Q = n•∆Hvap
Q = 3.118 × 43.3
Q = 135 KJ
Therefore, the heat required is 135 KJ
Select the topics that a student majoring in atmospheric science would be required to study.
mathematics
political science
physics
climatology
computer programming
physical geography
remote sensing
geographic information systems
Answers
Answer:
whats the answer//
Explanation:
Answer:
A,D,G,H
Explanation:
Mathematics
Climatology
Remote sensing
Geographic information systems
Phosphorus has greater ionization energy than sodium true or false?
Answers
It's true trust me gang^^^^
17. Find the average atomic mass unit of silicon given the following isotopes and
abundances: Silicon-28, Silicon-29, Silicon-30 with masses of 27.977, 28.976,
29.974, respectively. The percent abundance of the silicon isotopes is 92.2%, 4.7%
and 3.1%, respectively. Periodic table and it’s trends
Answers
The average atomic mass unit of Silicon : 28.0774
Further explanationGiven
Isotopes of Si(Silicon-28, Silicon-29, Silicon-30)
Required
The average atomic mass
Solution
Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
Avg. atomic mass = %mass.isotopes 1 + %mass.isotopes 2...etc
Input the values :
Avg atomic mass = (0.922 x 27.977) + (0.047 x 28.976) + (0.031 x 29.974)
Avg atomic mass = 28.0774
Number the elements sodium, magnesium, phosphorus, and chlorine in the predicted order ionization energies from the highest to the smallest. sodium magnesium phosphorus chlorine
Answers
Answer:
It goes 4 3 2 1
Explanation:
Alphabetical order
Answer:
4- sodium, 3-magnesium, 2-phosphorus, 1-chlorine
Explanation:
edge!
Rita determined the experimental van 't Hoff factor, i, for KCl to be 1.9 which is less than the theoretical value of 2. Select the option that best explains the difference between the theoretical and experimental i.a) The difference is due to the ion-pairing effect which effectively reduces the number of solute particles present in the solution.b) The difference is due to the ion-pairing effect which effectively increases the number of solute particles present in the solution correct amount of KCl that will give better agreement between the experimental and theoretical results.c) Rita did not freeze the entire sample.
Answers
Answer:
The difference is due to the ion-pairing effect which effectively reduces the number of solute particles present in the solution.
Explanation:
Colligative properties are those properties that depend on the amount of solute present. Such properties include; boiling point elevation, freezing point depression etc.
Ion pairing causes the Van't Hoff factor to deviate from whole numbers. Ion pairing effect generally reduces the number of solute particles in solution thereby decreasing the experimental value of the Van't Hoff factor (i).
Hence, the reason why Rita determined the Van't Hoff factor as 1.9 and not the theoretical value of 2 is because of on-pairing effect which effectively reduces the number of solute particles present in the solution.
The difference between the theoretical and experimental is A. The difference is due to the ion-pairing effect which effectively reduces the number of solute particles present in the solution.
Colligative propertiesIt should be noted that colligative properties simply means the properties that depend on the amount of solute present.
The ion pairing causes the Van't Hoff factor to deviate from whole numbers. Therefore, they caused the difference between the theoretical and experimental values.
Learn more about ion on:
https://brainly.com/question/11638999
1 mole = _____________________ _________________ particles
Answers
1 mole = 6.02 * 10^23 atoms
This is known as Avogadro's Number. This value is approximate.
Answer:
6.022×10^23
Explanation:
6.022×10^23 is known as avagadro's number or avagadro's constant
What is the name of the molecule below?HHOA. MethaneOB. MetheneO C. EthyneOD. EtheneC=CHH
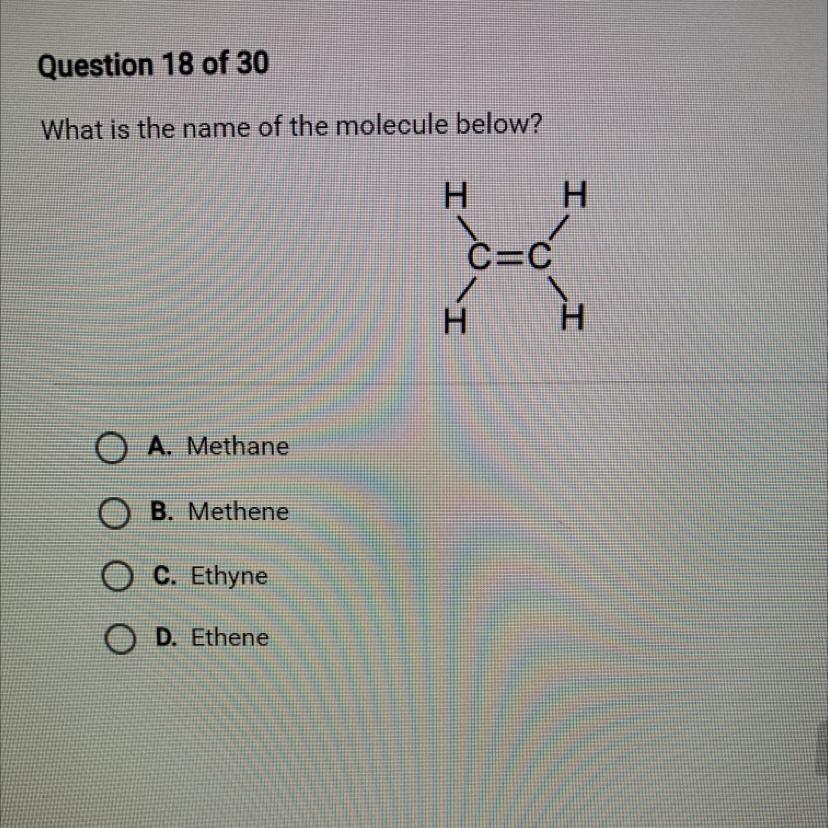
Answers
In this case, we have a two carbon molecule, the prefix for this type of molecule is Et
Since this compound has a double bond, the suffix will be ene
Therefore we have in this question, the molecule Ethene, letter D
Complete the chart on the characteristics of bonds
Answers
Answer:
Face value. Corporate bonds normally have a par value of $1,000, but this amount can be much greater for government bonds.
Interest. ...
Coupon or interest rate. ...
Maturity. ...
Issuers. ...
Rating agencies. ...
Tools and tips.