How long has it been that scientists have accepted that the nucleus of the atom consists of neutrons and protons?
Answers
About a century ago, in 1911, Rutherford discovered the atomic nucleus, then in 1919, he witnessed the proton.
For a long time, people believed that Atom was the final particle and the building block of every other component in the universe. However, in the year 1911, Rutherford's atomic model depicting the presence of nucleus shattered the argument that atom is the ultimate unbreakable particle.
Rutherford's experiment also approved that there must be more than just protons in the nucleus. For example, it was previously known that helium had an atomic number of 2, but a mass number of 4.
To know more about nucleus, refer to brainly.com/question/12602839
#SPJ4
Related Questions
LOOK AT THE IMAGE ABOVE CAN SOMEONE PLEASE DO IT WITH FULL STEPS PLEASE I NEED IT TODAY PLEASE PLEASE I WILL MARK YOU BRAINLIST

Answers
answer:
IMAGE IS SUPER UNCLEAR SORRY LUV <3
explanation:
your phone must me from 2009 with that trash camera quality sweetie i suggest you get a new one... oh wait you can't you're broke :(
Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
Answers
Answer:
This is a balanced equation because the same types of atoms and the same numbers of each atom are present on both sides of the equation. 8 S atoms are found on both sides of the equation, and 48 F atoms are found on both sides of the equation. S8 and 24F2 are the reactants; 8SF6 is the product. This is a combination reaction because two substances (S8 and 24F2) undergo a chemical union to form a more complex substance (8SF6).
S8+24F2→8SF6
Explanation:
Write a balanced radiochemical equation describing this process and the nuclide that forms when 206pb absorbs 4 neutrons. Include all superscripts and subscripts representing mass and charge
Answers
206Pb + 4n -> 210Pb
The process described is called nuclear capture, where the nucleus absorbs neutrons and transforms them. The nuclide produced when 206 Pb absorbs four neutrons is 210 Pb. The balanced radiochemical equation for this process can be written as
206Pb + 4n -> 210Pb
In this formula, the superscript indicates the mass number (the total number of protons and neutrons in the nucleus) and the subscript indicates the atomic number (the number of protons in the nucleus). The symbol 'n' stands for neutron..
Nuclear trapping is a type of nuclear reaction that occurs when the nucleus absorbs neutrons and undergoes transmutation. The nucleus either remains stable or becomes unstable and radioactively decays.
Read more about this on brainly.com/question/28810502
#SPJ4
Chemical Changes occur when the molecules of a substance are
(Blank) (Blank) and changed into a new substance with new (Blank)

Answers
Answer:
when molecules loose or gain electro it get charge and when it get attached with other charge molecules the form bond
ans is loose or gain ,bond
complete and balance the following neutralization reaction, name the products, and write the net ionic equation.
HBr (aq) + KOH (aq) --> KBr + H_2O
Answers
The neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH) can be completed and balanced as follows:
HBr(aq) + KOH(aq) → KBr(aq) + H2O(l)
The products of this reaction are potassium bromide (KBr) and water (H2O).
To write the net ionic equation, we exclude the spectator ions, which are the ions that appear on both sides of the equation unchanged. In this case, the potassium ion (K+) is a spectator ion because it appears on both sides. Therefore, the net ionic equation is:
H+(aq) + OH-(aq) → H2O(l)
In the net ionic equation, the hydrogen ion (H+) from the acid and the hydroxide ion (OH-) from the base combine to form water (H2O).
To know more about hydrobromic refer here
https://brainly.com/question/30459979#
#SPJ11
What structures are found in
plant cells that are not found in animal cells?
Answers
Answer:
Plant cells have a cell wall, chloroplasts and other specialized plastids, and a large central vacuole, which are not found within animal cells. The cell wall is a rigid covering that protects the cell, provides structural support, and gives shape to the cell.
Explanation:
MOST BRAINLIEST
How to find out if a solid, more specifically powdered cement, is acid or alkali?
Answers
To determine whether a powdered cement is an acid or an alkali, you can perform a simple pH test using litmus paper or a pH meter.
Acids have a pH value below 7, whereas alkalis have a pH value above 7.
To conduct a pH test using litmus paper, moisten the paper with water, then sprinkle a small amount of the powdered cement onto the paper. The paper will change color based on the pH of the cement. If the paper turns red, the cement is acidic. If it turns blue, the cement is alkaline.
Alternatively, you can use a pH meter to measure the pH of a solution made by mixing a small amount of the powdered cement with water. If the pH is less than 7, the cement is acidic, and if it is greater than 7, the cement is alkaline.
It is important to note that most types of cement are typically slightly alkaline, with a pH value between 8 and 9.5, due to the presence of calcium oxide and other alkali metal oxides in the cement.
To know more about pH test, refer here:
https://brainly.com/question/29551790#
#SPJ11
at the left.
6.
1. Work equals
A force x distance
B. force - distance
C. force + distance.
The force you apply to a machine
is the
A. input force.
B. output force.
C. efficiency.
2.
The unit of work is the
A. watt.
B. newton.
C. joule.
7.
The efficiency of all real
machines is
A. greater than 100%
B. equal to 100%
C. less than 100%
3.
Power is the amount of
A. work done per unit of time.
B. force on a certain area.
C. pressure in a volume of liquid.
8.
4.
The unit for power is the
A. joule.
B. Meter per second
C. watt.
The fixed point that a lever rotates
around is called the
A. fulcrum.
B. input force.
C. wedge.
9.
5.
A machine cannot change the
A. direction of the input force.
B. amount of work needed to do
A screwdriver is an example of a
simple machine called a
A. pulley.
B. screw
C. wheel and axle.
a task.
C. distance over which a force
is applied
10.
Your front teeth are
A. wedges.
B. levers.
C. compound machines.

Answers
Answer:
i know right answer
Explanation:
let me explain
5) If an atom has a positive charge what is it called?
Answers
Answer:
An atom that has a negative or positive charge it is called an ion.
Answer:
It''s called a Cation
Explanation:
Diffusion in Solids It is desired to calculate the rate of diffusion of CO₂ gas in air through a loosely packed bed of sand at 276K and a total pressure of 1 atm. The bed depth is 1.25 m and the void fraction e is 0.3. The partial pressure of CO₂ at the top of the bed is 2.026 x 10' Pa and 0 Pa at the bottom. Assume equimolar counterdiffusion of CO₂ and air. Use a t of 1.87. DAB-0.142×10 m²/s.
Answers
the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
To calculate the rate of diffusion of CO₂ gas in air through a bed of sand, we can use Fick's law of diffusion:
J = -DAB (dC/dx)
where J is the molar flux of CO₂, DAB is the diffusion coefficient of CO₂ in air, and (dC/dx) is the concentration gradient of CO₂ in the direction of diffusion.
To calculate the concentration gradient, we can use the following equation:
(dC/dx) = (ΔC/Δx)
where ΔC is the difference in partial pressure of CO₂ between the top and bottom of the bed, and Δx is the bed depth.
We are given that the bed depth is 1.25 m and the void fraction is 0.3, which means that the volume of the bed is:
V = (1 - e) A L
where A is the cross-sectional area of the bed and L is the bed depth. Assuming a circular cross-section, we can calculate the area as:
A = π (d/2)²
where d is the diameter of the bed. We are not given the diameter, so we cannot calculate the area.
However, we are given the partial pressure of CO₂ at the top and bottom of the bed, as well as the diffusion coefficient and temperature. We can use these values to calculate the molar flux of CO₂ using Fick's law of diffusion.
First, we need to convert the diffusion coefficient to the appropriate units:
DAB = 0.142 × 10^-9 m²/s
Next, we can calculate the concentration gradient:
ΔC = 2.026 × 10^4 Pa - 0 Pa = 2.026 × 10^4 Pa
Δx = 1.25 m
(dC/dx) = (ΔC/Δx) = (2.026 × 10^4 Pa/1.25 m) = 1.6208 × 10^4 Pa/m
Finally, we can calculate the molar flux of CO₂:
J = -DAB (dC/dx) = -(0.142 × 10^-9 m²/s) (1.6208 × 10^4 Pa/m) = -2.304 × 10^-6 mol/(m²·s)
The negative sign indicates that the molar flux of CO₂ is in the opposite direction of the concentration gradient, which is expected for equimolar counterdiffusion.
Therefore, the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
Why is air cooled before nitrogen and oxygen are obtained
Answers
Answer:
The main reason why air is cooled before nitrogen and oxygen are obtained is because these two gases are much more soluble in cold air than in warm air. As a result, if air were not cooled before these gases were separated, they would simply mix back together again.
If it takes 1.33 seconds for radio waves (which travel at the speed of light) to reach the moon from Earth, how far away is the moon?
Answers
Speed of light * time = distance
299,792,458 * 1.33 = 398,723,969 meters
398,723.969 km to the moon
If the radio waves from earth reach moon within 1.33 s in a speed of light then, the distance of moon from earth will be 3.9 ×10⁸ m.
What are radio waves?Radio waves are electromagnetic waves with highest wavelength and lesser energy. The speed of these waves will be equal to the light speed that is, 3 ×10⁸ m/s.
Distance is the product of time and velocity. It is given that the time taken by the radio wave to reach moon from earth is 1.33 seconds and the velocity of wave is 3 ×10⁸ m/s thus, distance to moon is calculated as follows:
Distance = Time × velocity
= 1.33 s × 3 ×10⁸ m/s
= 3.9 ×10⁸ m
Hence, moon is 3.9 ×10⁸ m away from earth.
To learn more about radio waves?
https://brainly.com/question/13989450
#SPJ2
Suppose that a beaker of water is 15°C and you raise the
temperature by 5°C. Use the graph above to calculate the percent decrease in the amount of dissolved O2 gas.

Answers
The percentage decrease in the amount of dissolved oxygen is 10%
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. If the actual and theoretical yield are the same, the percent yield is 100%
In chemistry, yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage.
From the graph,
The amount of dissolved oxygen at 15°C is 10 mg/L
The amount of dissolved oxygen at 20°C is 9 mg/L
The decrease in the amount of dissolved oxygen is 1mg/L
The percentage decrease = (1/10) × 100 = 10%
Learn more about Percentage yield, here:
https://brainly.com/question/30794636
#SPJ1
an isotope of gallium, 67ga, has an atomic number of 31 and a half-life of 78 hours. consider a small mass of 3.2 grams for 67ga which is initially pure. 1)initially, what is the half-life of the gallium? t1/2o
Answers
The half-life is a constant property of an isotope and does not change based on the mass or purity of the sample.
The initial half-life of 67Ga is given as 78 hours. This means that after 78 hours, the mass of 67Ga will be reduced to half of its initial value. Gallium-67 (67Ga) is an isotope of gallium with an atomic number of 31 and a half-life of 78 hours. When considering a small mass of 3.2 grams of initially pure 67Ga, the initial half-life (t1/2o) remains the same as the half-life of this particular isotope, which is 78 hours. The half-life is a constant property of an isotope and does not change based on the mass or purity of the sample. When considering a small mass of 3.2 grams of initially pure 67Ga, the initial half-life (t1/2o) remains the same as the half-life of this particular isotope, which is 78 hours.
To know more about isotope visit:
https://brainly.com/question/28039996
#SPJ11
4 molecules of hydrogen (H2) react with 2 molecules of oxygen (O2) to produce some amount of water (H2O).
Complete the table below.
Chemical element Number of atoms in the reaction
-H -
-O -
During this reaction, how many molecules of water (H2O) are produced?
Answers
Answer:
2H2+ 02-->2H20
So 4H2 + 202-->4H20
so the answer is 4 water molecules.
Diphosphorus pentoxide(P2O5) reacts with water to form phosphoric acid, a major industrial acid. In the laboratory, the oxide is used as a drying agent. a.What is the mass of 4.65 x 10 22molecules of phosphorus pentoxide?
Answers
Answer: The mass of \(4.65\times 10^{22}\) molecules of phosphorus pentoxide is 20.5 g
Explanation:
According to avogadro's law, 1 mole of every substance weighs equal to the molecular mass and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given molecules}}{\text {avogadro's number}}=\frac{4.65\times 10^{22}}{6.023\times 10^{23}}=0.0772moles\)
1 mole of \(P_2O_5\) weigh = 283.9 g
Thus 0.0772 moles of \(P_2O_5\) weigh = \(\frac{283.9}{1}\times 0.0722=20.5g\)
Thus the mass of \(4.65\times 10^{22}\) molecules of phosphorus pentoxide is 20.5 g
How many molecules are there in 4.00 moles of glucose, C6H1206?
Answers
Molecules in 4 moles of glucose are 24.088x10²³.
We need to find the number of molecules by applying the concept of moles
number of moles(n)= Number of Molecules(N)/Avogadro's Number(Nₐ)
n=N/Nₐ
4=N/6.022x10²³
N=24.088x10²³
Therefore, the number of molecules in 4 moles of Glucose is 24.088x10²³.
To know more about moles, click on https://brainly.com/question/15356425
Which of these would be the best order for arranging the materials from biggest to smallest?
Talcum powder, salt, gravel, soil
Gravel, salt, soil, talcum powder
Gravel, talcum powder, salt, soil
Salt, gravel, powder, soil
Answers
The correct order of the materials from the biggest to smallest size is option b, gravel, salt, soil and talcum powder.
What is gravel ?Gravel is the aggregates of less rounded rocks. In some locations, heavy metallic ore deposits like cassiterite or native metals like gold, in the form of nuggets or flakes, can be found accumulating in gravel beds. Gravel is a common building material.
Therefore, the gravel is the biggest here. Salt in nature appears as salt rocks. Soil is some more grained. Talcum powder is very fine and tiny soft particles. Hence, talcum powder is the smallest here.
Therefore, the order of materials from biggest to smallest is option b, gravel, salt, soil and talcum powder.
Find more on talcum powder :
https://brainly.com/question/29940887
#SPJ1
Why is this canyon an example of how the surface of Earth changes slowly map test question
Answers
This canyon is an example of how the surface of Earth changes slowly due to the slow and gradual process of erosion by water and wind.
What process is responsible for the slow change in the surface of Earth demonstrated by the canyon in the given question?The slow and gradual process of erosion by water and wind is responsible for the slow change in the surface of Earth demonstrated by the canyon.
How does the canyon serve as an example of the slow change in the surface of Earth?The canyon demonstrates how the surface of Earth changes slowly due to the process of erosion by water and canyon, which gradually wear away the rock and reshape the landscape over millions of years.
Learn more about canyon here:
https://brainly.com/question/10770602
#SPJ1
A change of matter from one form to another without changing its chemical properties ?
Options:
Physical change
Chemical change
Answers
Explanation:
A physical change does not change the chemical properties of a compound. Instead a change in state occurs, for example evaporation where a liquid becomes a gas.
Answer:
Physical change
Explanation:
A physical change is a change of matter from one form to another without a change in chemical properties.
1 point
If the element Lithium (Li) were to bond with the element Sulfur (s), what
type of bond can you predict will be formed and why?*
A. Covalent-Li and S are two nonmetals
B. Covalent-Li is a metal and S is a nonmetal
C. Ionic-Li and S are two nonmetals
D. Ionic-Li is a metal and S is a nonmetal
Answers
Answer:
C. Ionic-Li and S are two nonmetals
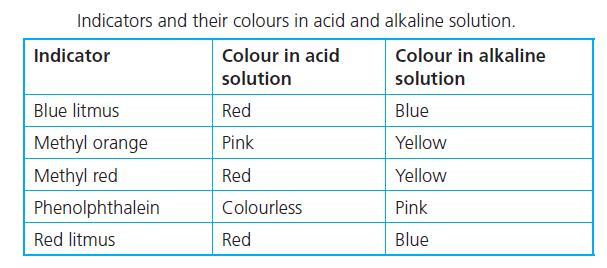
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
HELP ASAP!! 12pts!
What is the molarity of an aqueous solution containing 7.5 moles of
calcium chloride in 12.75 L of solution?
A. 0.59 M
B. 1.7M
C. 7.5M
D. 95.6 M
Answers
Answer:
The correct answer is 0.59M
1.18 g of sodium chloride is added to 21.8 mL of water. Calculate the theoretical molality of the solution. mol/kg
Answers
The theoretical molality of the sodium chloride solution is 0.926 mol/kg.
Steps are:
1. Convert the mass of sodium chloride to moles.
The molar mass of sodium chloride (NaCl) is 58.44 g/mol.
moles of NaCl = (mass of NaCl) / (molar mass of NaCl)
moles of NaCl = 1.18 g / 58.44 g/mol = 0.0202 mol
2. Convert the volume of water to mass.
Assume that the density of water is 1 g/mL.
mass of water = (volume of water) × (density of water)
mass of water = 21.8 mL × 1 g/mL = 21.8 g
3. Convert the mass of water to kilograms.
mass of water in kg = mass of water in g / 1000
mass of water in kg = 21.8 g / 1000 = 0.0218 kg
4. Calculate the molality.
molality = moles of solute / mass of solvent (in kg)
molality = 0.0202 mol / 0.0218 kg = 0.926 mol/kg
The theoretical molality of the sodium chloride solution is 0.926 mol/kg.
Learn more about molality here:
https://brainly.com/question/31645632
#SPJ11
which statement accurately describes the charge of the nucleus of an atom? - the nucleus can be either positively charged or neutral. - the nucleus never has an electrical charge. - the nucleus always has a positive charge. - the charge of a nucleus can change from a positive to a negative.
Answers
Answer:
the nucleus always has a positive charge
Explanation:
The nucleus of an atom is comprised of at least one proton and zero or more neutrons. Neutrons have no charge, but protons have a positive charge. Thus, the net charge of a nucleus is always positive.
small airborne particles of solid substances such as grain, flour, sugar, coal, metal, or sawdust:
Answers
Small airborne particles of solid substances such as grain, flour, sugar, coal, metal, or sawdust are commonly known as dust.
Dust is an accumulation of small particles that are released into the air through various activities such as cutting, grinding, drilling, or blasting. These activities generate a lot of fine dust particles, which can cause respiratory problems when inhaled. The type of dust generated depends on the source material and the nature of the activity. For example, sawdust is generated during woodworking, flour dust is common in bakeries, and coal dust is generated in coal mines.
Dust particles are classified based on their size, with the smallest particles being the most dangerous. Fine dust particles, also known as PM2.5, are small enough to penetrate deep into the lungs and can cause a range of health problems such as asthma, bronchitis, and lung cancer. Long-term exposure to dust can also lead to chronic respiratory diseases. Dust control measures such as ventilation, dust suppression, and personal protective equipment can help reduce the risk of dust exposure in workplaces.
Learn more about bronchitis here :
https://brainly.com/question/31024710
#SPJ11
Stoichiometry is the branch of chemistry that deals with elements in
compounds and with reactants and products in chemical reactions,
focusing on.
a. bonding
b. energy transfers
c. mass relationships
d. physical characteristics
Answers
C. Mass relationships
Explanation: hope this helped
I got it right on the test! :)
40. 0% carbon, 6. 7% hydrogen, and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?
Answers
The molecular formula of the unknown compound is C2H2O2.
To determine the molecular formula of the unknown compound, we need to calculate the empirical formula first and then find the multiple of its subscripts to obtain the molecular formula.
Given:
Percentage of carbon = 40.0%
Percentage of hydrogen = 6.7%
Percentage of oxygen = 53.3%
Molecular mass = 60.0 g/mol
Step 1: Convert the percentages to grams.
Assuming we have 100 grams of the compound:
Mass of carbon = 40.0 g
Mass of hydrogen = 6.7 g
Mass of oxygen = 53.3 g
Step 2: Convert the masses to moles using the molar masses of the elements.
Molar mass of carbon = 12.01 g/mol
Molar mass of hydrogen = 1.008 g/mol
Molar mass of oxygen = 16.00 g/mol
Number of moles of carbon = Mass of carbon / Molar mass of carbon
= 40.0 g / 12.01 g/mol
= 3.332 mol
Number of moles of hydrogen = Mass of hydrogen / Molar mass of hydrogen
= 6.7 g / 1.008 g/mol
= 6.648 mol
Number of moles of oxygen = Mass of oxygen / Molar mass of oxygen
= 53.3 g / 16.00 g/mol
= 3.331 mol
Step 3: Determine the empirical formula by dividing the moles by the smallest value.
Dividing the moles of carbon, hydrogen, and oxygen by 3.331 gives approximately 1 for each element.
So, the empirical formula of the compound is CHO.
Step 4: Determine the multiple of the subscripts to obtain the molecular formula.
To find the multiple, we divide the molecular mass by the empirical formula mass.
Molecular mass = 60.0 g/mol
Empirical formula mass = (12.01 g/mol) + (1.008 g/mol) + (16.00 g/mol) = 29.018 g/mol
Multiple = Molecular mass / Empirical formula mass
= 60.0 g/mol / 29.018 g/mol
= 2.07
Rounding to the nearest whole number, we get 2.
Therefore, the molecular formula of the unknown compound is C2H2O2.
learn more about molecular here
https://brainly.com/question/30640129
#SPJ11
For this specific titration, we took 52mL of NaOH and put it in the burette and had it added to 25mL of an unknown acetic acid. We found that the acid turned pink after 9mL of the NaOH was added. This is the question:
How many moles of NaOH were necessary to reach the equivalence point? Choose the closest answer. Select one: a. 3.26 x 10-4 moles b. 9.09 x 104 moles c. 4.67 102 moles d. 5.02 x 10 moles
Answers
The number of moles of NaOH is 9 × 10⁻⁴.
Generally, one mole of any substance is defined as value that is equal to the value of 6.023 x 10²³ (Avagadro number). It can be basically used to measure the products obtained from the chemical reaction. The unit of the mole is basically denoted by mol.
Concentration of NaOH,
M = W/Gmw × 1000/V(mL)
M = 1/40 × 1000/250
M = 1/40 × 4 = 1/10
M = 0.1 moles
But according to the question the acid turned pink after 9mL of the NaOH was added so number moles of moles of NaOH needed to reach equivalence point.
n = M × V(lit)
n = 0.1 × 9 mL/1000
n = 9 × 10⁻⁴ moles
Learn more about moles from the link given below.
https://brainly.com/question/26416088
#SPJ4
what are things that we use that are made of the elements on the periodic table
Answers
Answer:
all matter (everything) is made of the elements on the periodic table
Explanation: