Answers
Answer:
4.5 × 10²³ number of atoms Al
Explanation:
Given data:
Mass of Al = 20 g
Number of atoms of Al = ?
Solution:
The given problem will solve by using Avogadro number.
'It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance" .The number 6.022 × 10²³ is called Avogadro number.
We will calculate the number of moles of Al first:
Number of moles = mass/molar mass
Number of moles = 20 g/ 27 g/mol
Number of moles = 0.74 mol
1 mole = 6.022 × 10²³ number of atoms
0.74 mol × 6.022 × 10²³ number of atoms / 1 mol
4.5 × 10²³ number of atoms Al
The required number of atoms is \(4.46\times 10^{23}\)
Atomic Weight:Atomic weight, also called relative atomic mass, ratio of the average mass of a chemical element’s atoms to some standard.
Given that,
Mass=\(20\) grams
1 mole of aluminum \(= 27g = 6.023\times10^{23}\) number of atoms 27g of aluminum contains \(6.023\times10^{23}\) a number of atoms.
Therefore,20 grams the number of atoms is,
\(6.023\times10^{23}\times\frac{20}{27} =4.46\times10^{23}\)
Learn more about the topic of atomic weight:
https://brainly.com/question/13399225
Related Questions
This planet is about the size of Earth. It has a very thick atmosphere with
a lot of gases. The surface temperatures and pressures are extremely
high.
Which planet does this describe?

Answers
Answer:
Venus
Explanation:
Mars has a thin atmosphere
Saturn is really cold (-288F)
Mercury has no atmosphere
Enter your answer in the provided box. Acrylic acid (CH2═CHCOOH) is used to prepare polymers, adhesives, and paints. The first step to make acrylic acid involves the vapor-phase oxidation of propylene (CH2═CHCH3) to acrolein (CH2═CHCHO). This step is carried out at 330°C and 2.50 atm in a large bundle of tubes around which circulates a heat-transfer agent. The reactants spend an average of 1.80 s in the tubes, which have a void space of 92.1 ft3. How many pounds of propylene must be added per hour in a mixture whose mole fractions are 0.0700 propylene, 0.3500 steam, and 0.5800 air?
Answers
Answer: m = 1710.35 pounds per hour
Explanation: Since the step involves a gaseous state, it can be used the ideal gas formula, given by:
PV=nRT
For this question, R will be 8.31451 m³Pa/K.mol, which means the variables need a change of unit:
T = 330°C + 273 = 603K
P = 2.5atm*101325 = 253312.5Pa
V = 92.1ft³*0.0283 = 2.60643m³
Calculating mols:
PV=nRT
\(n=\frac{253312.5*2.60643}{8.31451*603}\)
n = 131.7 mols
The reactant (propylene) spends 1.80 seconds in the tubes and 1 hour is 3600 seconds, so:
\(\frac{131.7*3600}{1.8}\) = 263377.5 mol/h
In the mixture, the proportion of propylene is 0.07, then:
263377.5*0.07 = 18436.4 mol of propylene in the mixture
Mol is related to mass and molar mass by the following formula:
\(n=\frac{m}{M}\)
m = nM
Molar Mass of propylene is 42.08g/mol:
m = 18436.4*42.08 = 775803.712g
Changing into pounds:
\(m=\frac{775803.712}{453.6}\)
m = 1710.35lbs
It must be added 1710.35lbs per hour in the mixture.
Can someone help me please!!

Answers
Answer:
1. AgNO₃ (aq) + NaCl (aq) ----> NaNO₃ (aq) + AgCl (s)
2. Li₂SO₄ (aq) + BaCl₂ (aq) ----> 2 LiCl (aq) + BaSO₄ (s)
3. 2 NaOH (aq) + MgCl₂ (aq) ----> 2 NaCl (aq) + Mg(OH)₂ (s)
Explanation:
The reaction involving the mixing of two soluble solutions to produce a precipitate is known as a precipitation reaction.
A precipitation reaction is double-replacement reaction (a reaction that exchanges the cations or the anions of two ionic compounds) in which one product is a solid precipitate.
Precipitation reactions at useful in the identification of various ions present in a solution. In order to predict the reactions that will produce a precipitate, solubility rules as given in the solubility table below can be used.
From the tables, the reactions that will produce a precipitate, as well as their balanced molecular equations are as follows:
1. AgNO₃ (aq) + NaCl (aq) ----> NaNO₃ (aq) + AgCl (s)
2. Li₂SO₄ (aq) + BaCl₂ (aq) ----> 2 LiCl (aq) + BaSO₄ (s)
3. 2 NaOH (aq) + MgCl₂ (aq) ----> 2 NaCl (aq) + Mg(OH)₂ (s)

How many calories of energy are found in 8.3 grams or Fat? Every gram of fat
can produce 38 KJ of energy.
Answers
Answer: asd asd asd asdasdasdasd
place the following carboxylic acid derivatives in order of increasing reactivity toward nucleophiles, starting with the least reactive at the top of the list. EsterAcid chloide
AmideAcid anhydride
Answers
Acid Chloride, followed by Acid Anhydride, Acid Ester, and Acid Amide, is the sequence of reactivity for carboxylic acid derivatives. Acid amides are the least reactive, while acid chlorides are more so.
This is as a result of the leaving group's basicity order, which is as follows.
Acid Chloride, followed by Acid Anhydride,, Acid Ester, and Acid Amide, is the sequence of reactivity for carboxylic acid derivatives. Acid amides are the least reactive, while acid chlorides are more so. This is as a result of the leaving group's basicity order, which is as follows. Was this response useful? The following carboxylic acids should be arranged in decreasing order of reactivity. The carboxylic acid must first be activated before a nucleophilic substitution may take place. Acid chlorides can be transformed into amides, esters, or acid anhydrides. Because acid chlorides are the most reactive of the compounds, these reactions are feasible.
To learn more about Acid Anhydride, please click on below link
https://brainly.com/question/4769772
#SPJ4
Look at picture and answer will mark brainliest.
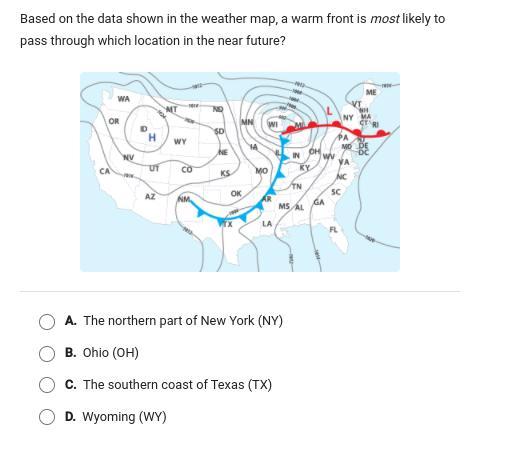
Answers
Answer:
A
Explanation:
10. What is the molality of a solution
containing 288 g of calcium chloride
dissolved in 2.04 kg of water?
Answers
The choice of solution has a concentration of 1.144 mol/kg molality.
What exactly are molality and molarity?Molarity corresponds to the moles of solvent divided by the amount of solution in litres, whereas molality is equal with the moles of solvent divided by the quantity of solvent in kilogrammes.
Is one molarity the same as one molality?Since 1 mole of solute is present in 1 litre for the solution, which contains both the solute and the solvent, 1 molar aqueous solutions are more concentrated than one decays aqueous solutions.
To know more about molality visit:
https://brainly.com/question/26921570
#SPJ1
Part B
When carbon is burned in air, it reacts with oxygen to form carbon dioxide. When 15.6 g of carbon were burned in the presence
of 52.1 g of oxygen, 10.5 g of oxygen remained unreacted. What mass of carbon dioxide was produced?
Answers
When the Carbon reacts with oxygen it produce CO₂. This can be depicted by the below equation.
C + O₂ → CO₂. By the given process, 57.2 g of CO₂ are produced.
It has been mentioned that when 15.6 g of C reacts with 52.1 g of O₂ , then 10.5 g of O₂ remains unreacted. It indicates that Carbon is the limiting reagent and hence the amount of CO₂ produced is based on the amount of Carbon burnt.
C + O₂ → CO₂
In the given equation , 1 mole of carbon reacts with the 1 mole of O₂ to produce 1 mole of CO₂.
In the case 15.6 g of Carbon reacts with 52.1 of O₂ to produce the "x" g of CO₂.
No of moles of a substance = mass of the substance/molar mass of substance
No of moles of carbon = 15.6 /12= 1.3 moles
No of moles of O₂ = Mass of reacted O₂/Molar mass of O₂.
No of moles of O₂ = (Total mass of O₂ burned - Mass of unreacted O)/32
No of moles of O₂ = (52.1-10.5) ÷ 32 = 1.3 moles.
Hence as already discussed 1 mole of Carbon reacts with 1 mole of O₂ to produce 1 mole of CO₂. In this case 1.2 moles of carbon reacts with 1.3 moles of O₂ to produce 1.3 moles of CO₂.
Moles of carbon dioxide = Mass of CO₂ produced /Molar mass of CO₂
Mass of CO₂ produced(x) = Moles of CO₂ ×Molar mass of CO₂
Mass of CO₂ produced(x) = 1.3 x 44 = 57.2 g
Thus 57.2 g of CO₂ is produced.
To know more about formation of CO₂ , please refer:
https://brainly.com/question/15030226
#SPJ1
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8
Can the ph scale be utilized for all acids (Arrhenius, Bronsted-Lowery, and Lewis)? Explain reasoning.
Answers
Answer:
No, the pH scale can not be utilized for all types of acids known
Explanation:
The pH scale is a scale that shows the degree of acidity or alkalinity of a substance. The pH scale is graduated from 0-14. The pH scale is mostly applied to Arrhenius acids. Recall that in the Arrhenius definition, an acid produces hydrogen ion in solution while a base produces hydroxide ion in solution. Hence we could easily measure or calculate the pH and pOH of Arrhenius acids. Arrhenius acids apply mostly to acid-base discussion in Aqueous media.
Beyond aqueous media, we can still apply the Brownstead-Lowry and Lewis definitions for acid and base. Some of these substances to which we apply these other definitions of acid and base do not necessarily contain hydrogen or hydroxide ions hence the pH scale can not be utilized in discussing their acid-base properties, hence the answer.
What is the molarity of a solution containing 2 moles of sodium hydroxide (NaOH) dissolved in 4.0 L of solution?
Answers
Answer:
0.5M
Explanation:
Molarity = Mols/ Liters
so 2/4
0.5 M
exothermic reaction in which the reaction releases energy as heat. 4. How would the reaction would change if you added 2 g of steel wool to the acetate and reduced the iron acetate solution down to 50 mL (evaporated off 100 mL).
Answers
Answer:
Explanation:
We know that the composition of Steel includes Carbon and Iron. So, if 2g of steel wool was added to the iron acetate, then the iron acetate is now being reduced. What will happen is that the solution will be triggered as it becomes denser and get highly concentrated. Hence, an increase in concentration leads to a decrease in reaction rates. Thus, a slow reaction will take place, and the reaction rate will decrease.
An organic compound is made up of 0.72 g of carbon, 0.16 g of hydrogen and 0.32 g of oxygen. Calculate the empirical formula of the compound.
Answers
The Empirical formula of the compound is \(C_{6} HO_{2}\)
Given that,
Carbon \(=0.72g\)
Hydrogen \(=0.16g\)
Oxygen \(=0.32g\)
So the mole of the elements can be calculated as,
Carbon - \(\frac{0.72}{0.16} =4.5\) ≈ 6
Hydrogen - \(\frac{0.16}{0.16} =1\)
Oxygen - \(\frac{0.32}{0.16} = 2\)
∴The Empirical formula of the compound is \(C_{6} HO_{2}\)
To learn more about the Empirical formula visit:https://brainly.com/question/29068419
#SPJ1
What might have been the advantages and disadvantages of just having
experienced polar explorers at the catlin arctic survey.
Answers
While having experienced polar explorers at the Catlin Arctic Survey would have been beneficial in many ways, it would also have been important for them to work collaboratively with the rest of the team.
Advantages:
Experienced polar explorers would have had a wealth of knowledge and skills, such as how to travel over the ice, how to set up camp, and how to handle emergencies.
Experienced polar explorers would have been able to make informed decisions about the best routes to take and the most efficient ways to travel. This could have helped to save time and energy.
Disadvantages:
Experienced polar explorers may have been set in their ways and resistant to new ideas. This could have hindered the team's ability to adapt to changing circumstances and make the most of new opportunities.
Experienced polar explorers may have been overconfident and taken risks that the rest of the team was not comfortable with. This could have put everyone's safety at risk.
To learn more about the Catlin Arctic Survey, follow the link:
https://brainly.com/question/28313250
#SPJ1
What is the function of a lyase enzyme?
a. To assist the substrate by binding to the enzyme, enabling substrate to active site engagement
b. To facilitate a reaction of one substrate to form two products with the use of water
c. To facilitate a reaction of one substrate to form two products without the use of water
d. To tell fibs
Answers
Answer:
c. To facilitate a reaction of one substrate to form two products without the use of water
Explanation:
A lyase is an enzyme that catalyzes - accelerates the chemical reaction - in which a substrate is broken into two molecules. The reaction does not involve hydrolysis or oxidation, so the water molecule is not included in the chemical reaction. Thus, the enzyme facilitates the reaction in which a molecule (substrate) is decomposed into two molecules with the elimination of chemical bonds.
Which two nuclides are isotopes of the same element?
K and K
19
O
19
14C and ¹4N
20Na and 20 Ne
10
39K and 40 Ca
19
20
Answers
Answer:
19K and 19 K
Explanation:
The term "isotope" refers to an atom of the same element with the same number of protons but a different number of neutrons. So, despite having the same atomic number (Z), isotopes have differing mass numbers (A). In other words, elements with differing atomic numbers (protons) are not isotopes.
To know more about atom,
https://brainly.com/question/17545314
#SPJ4
The following balanced equation shows the formation of ammonia.
N2 + 3H2 Right arrow. 2NH3
How many moles of nitrogen are needed to completely convert 6.34 mol of hydrogen?
Answers
Answer:
For completely converting 6.34 moles of hydrogen to ammonia, 2.11 moles of Nitrogen is required.
The chemical reaction for the formation of ammonia by nitrogen and hydrogen reaction has been as follows:
For the formation of 2 moles of ammonia, 3 moles of hydrogen, and 1 mole of nitrogen s required.
The utilization of 3 moles of hydrogen requires 1 mole of Nitrogen.
So, the utilization of 6.34 moles of hydrogen requires:
3 moles Hydrogen = 1 -mole Nitrogen
6.34 moles hydrogen = moles of Nitrogen
6.34 moles of hydrogen requires = 2.11 moles of Nitrogen.
For completely converting 6.34 moles of hydrogen to ammonia, 2.11 moles of Nitrogen is required.
For more information about the ammonia formation, refer to the link:
brainly.com/question/20986532
rating answer section
Answer rating5.0
(20 votes)
A hypothetical AX type of ceramic material is known to have a density of 3.15 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.41 nm. The atomic weights of the A and X elements are 90.5 and 37.3 g/mol, respectively. On the basis of this information, which one of the following crystal structures is possible for this material?
a. Sodium chloride
b. Cesium chloride
c. Zinc blende
Answers
Answer:
b.
Explanation:
From the given information:
\(p = \dfrac{zM}{N_oa^3}\)
where;
a = 0.41 nm = \(4.1 \times 10^{-8} \ cm\)
M = 90.5 + 37.3 = 127.8 g/mol
\(N_o = 6.023 \times 10 ^{23}\)
p = 3.15 g/cm³
∴
\(3.15 = \dfrac{z \times 127.8}{(6.02 \times 10^{23}) \times (4.1 \times 10^{-8} )^3}\)
Z = 1
Thus, the ceramic material has a simple cubic crystal system and the crystal structure is possible to be Cesium chloride.
After mixing for three hours, the product is extracted into dichloromethane and the solvent is removed to give 245 mg of an oil. Using the moles of our protected aldehyde calculated earlier (2.96) and the molecular weight of the product (102 g/mol) predict the theoretical 100% yield of the product in milligrams. Round to the tenths place.
Answers
Answer:
the theoretical 100% yield of the product in milligrams is 302920 mg
Explanation:
Given the data in the question;
245 mg of an oil
Using the moles of our protected aldehyde calculated earlier (2.96)
the molecular weight of the product (102 g/mol) = 102000 mg/mol
so, the mass of aldehyde produced (100% yield) will be;
⇒ number of moles × molar mass
⇒ 2.96 mol × 102000 mg/mol
⇒ 302920 mol.mg / mol
⇒ 302920 mg
Therefore, the theoretical 100% yield of the product in milligrams is 302920 mg
A mixture of water and water vapor is present in a closed vessel at 20 degrees Celsius. the total pressure of the system is 755.0 mmHg. Partial pressure of water vapor at 20 degrees Celsius equals 17.5 mm Hg. What is the partial pressure of H2
Answers
Answer:
737.5 mmHg
Explanation:
Ptotal=PH2O+PH2
P is the pressure and since we have Ptotal and PH2O, we can solve for PH2
755=17.5+x
755-17.5=x
x=737.5
Classify the substances as atomic elements, molecular elements, molecular compounds, or ionic compounds.

Answers
Answer:
compounds ok I think I can't anderstand good
Why would a table be more suitable than a graph to show data
Answers
Tables are more suitable than a graph to show data because they present data in as close to raw form as possible.
How can data be represented?Data can be represented in Tables, charts and graphs. These data representation are used for two broad purposes.
The first is to support the collection, organization and analysis of data as part of the process of a scientific study. The second is to help present the conclusions of a study to a wider audienceUnlike the case of graphs that use abstraction to focus on trends and numerical relationships, tables present data in as close to raw form as possible.
Tables are usually meant to be read, so they are ideal when the data that is present are those type of data that cannot easily be presented visually, or when the data requires more specific attention.
Learn more about tables at: https://brainly.com/question/12151322
#SPJ1
]All organic compounds contain the element carbon but, not all compounds containing the element “carbon”are organic .Justify this statement.
Answers
The statement "All organic compounds contain the element carbon, but not all compounds containing the element 'carbon' are organic" can be justified based on the definition and characteristics of organic compounds.
Organic compounds are compounds primarily composed of carbon and hydrogen atoms, often with other elements like oxygen, nitrogen, sulfur, and phosphorus. These compounds are typically associated with living organisms and are known for their unique properties and behavior, including the ability to form complex structures, exhibit covalent bonding, and undergo organic reactions.
On the other hand, there are compounds that contain carbon but are not classified as organic. One notable example is carbon dioxide (\(CO_{2}\)), which is a simple inorganic compound composed of carbon and oxygen. Carbon dioxide does not possess the characteristic properties of organic compounds, such as the ability to form long chains or undergo organic reactions.
Additionally, there are inorganic compounds like carbonates (such as calcium carbonate) and carbides (such as calcium carbide) that contain carbon but are not considered organic. These compounds have distinct chemical and physical properties different from those of organic compounds.
In summary, while all organic compounds contain carbon, not all compounds containing carbon are organic. The classification of a compound as organic or inorganic depends on its overall molecular structure, bonding, and characteristic properties.
Know more about molecular structure here:
https://brainly.com/question/27789666
#SPJ8
1. What is the percent of NaCl in a mixture that contains 23.5 g of NaCl and 212 g of water? Enter
answers in 2 decimal places
Answers
Answer:
9.98%
Explanation:
To find the percent of NaCl in the mixture, we need to divide the mass of NaCl by the total mass of the mixture, and then multiply by 100 to express it as a percentage.
Step 1: Find the total mass of the mixture
total mass = mass of NaCl + mass of water
total mass = 23.5 g + 212 g
total mass = 235.5 g
Step 2: Calculate the percent of NaCl
% NaCl = (mass of NaCl / total mass) x 100
% NaCl = (23.5 g / 235.5 g) x 100
% NaCl = 0.0997876857 x 100
% NaCl = 9.978768677%
% NaCl = 9.98%
Therefore, the percent of NaCl in the mixture is 9.98%.
How many grams in 1.61 x 1023 molecules of water (H2O)
Answers
Taking into account the definition of Avogadro's number and molar mass, 4.806 grams of water are present in 1.61×10²³ molecules.
Definition of Avogadro's NumberAvogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023×10²³ particles per mole. Avogadro's number applies to any substance.
Definition of molar massThe molar mass of substance is a property defined as its mass per unit quantity of substance, in other words, molar mass is the amount of mass that a substance contains in one mole.
Mass of waterTaking into account the definition of Avogadro's Number, you can apply the following rule of three: if 6.023×10²³ molecules are contained in 1 mole of water, then 1.61×10²³ molecules are contained in how many moles of water?
amount of moles of water= (1.61×10²³ molecules× 1 mole)÷ 6.023×10²³ molecules
amount of moles of water= 0.267 moles
Now, taking into account the definition of molar mass, and knowing that the molar mass of water is 18 g/mole, you can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 18 grams, 0.267 moles of the compound contains how much mass?
\(mass= \frac{0.267 molesx 18 grams}{1 mole}\)
mass= 4.806 grams
Finally, 4.806 grams of water are present in 1.61×10²³ molecules.
Learn more about
Avogadro's Number:
brainly.com/question/11907018
brainly.com/question/1445383
brainly.com/question/1528951
molar mass:
brainly.com/question/5216907
brainly.com/question/11209783
brainly.com/question/7132033
brainly.com/question/17249726
#SPJ1
A pure substance is a single kind of matter. A(n) _________
is made up of two or more substances that are together in the same place, but each substance keeps its own properties.
Answers
A pure substance is indeed a single kind of matter with a uniform and definite composition. In contrast, a mixture is made up of two or more substances that are together in the same place, but each substance maintains its own properties.
Mixtures can be classified as either homogeneous or heterogeneous. A homogeneous mixture has a uniform composition throughout, meaning its components are evenly distributed and not easily distinguishable. Examples include solutions, such as saltwater or air.
On the other hand, a heterogeneous mixture has an uneven distribution of its components, and its individual substances can be easily identified. Examples include sand and water, or oil and water.
One key distinction between a pure substance and a mixture is that a pure substance has a fixed composition, while the composition of a mixture can vary. This means that mixtures can be separated into their individual components through physical processes like filtration, evaporation, or distillation, without undergoing any chemical changes.
In summary, a pure substance is a single type of matter with a definite and uniform composition, while a mixture consists of two or more substances that are combined in the same place, but each substance retains its individual properties. Mixtures can be homogeneous or heterogeneous and can be separated into their components through physical methods.
Know more about Mixtures here:
https://brainly.com/question/24647756
#SPJ11
Platinum can be found in which of the following formations
A.Sedimentary Bands
B. Aggregates
C. Chromite Bands
D. Hydrothermal Veins
Answers
Answer:
Explanation:
D
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Help plzzz I don’t get to

Answers
two uses of sodium carbonate
Answers
Sodium carbonate, also known as washing soda or soda ash, has a wide range of applications. Sodium carbonate can be naturally occurring or synthetically produced through various methods, including the Solvay process, which is the most common method of industrial production.
Sodium carbonate, also known as washing soda or soda ash, has many uses, including:
1) Cleaning agent: Sodium carbonate is an effective cleaning agent due to its alkaline nature. It is used in laundry detergents and household cleaners to remove stains and grease from clothes and surfaces.
2) Industrial applications: Sodium carbonate is used in a variety of industrial applications. It is used in the production of glass, pulp and paper, and soaps and detergents. It is also used as a water softener and pH regulator in chemical processes.
Learn more about Sodium Carbonate at
brainly.com/question/31344166
#SPJ1