Answers
The volume of the water in cubic meter is determined as 3.2 x 10⁶ m³ .
Weight of one gallon of water
The weight of 1 gal of water is given as 3785 g
Mass of 8.48 x 10⁸ gal = 3785 x 8.48 x 10⁸ = 3.2 x 10¹² g
Volume of the water in cubic metersVolume = mass/density
Volume = 3.2 x 10¹² g/1 gmL
Volume = 3.2 x 10¹² mL x 10⁻⁶ m³/mL = 3.2 x 10⁶ m³
Thus, the volume of the water in cubic meter is determined as 3.2 x 10⁶ m³ .
Learn more about volume here: https://brainly.com/question/1972490
#SPJ1
Related Questions
Hydrochloric acid is widely used as a laboratory reagent, in refining ore for the production of tin and tantalum, and as a catalyst in organic reactions. Calculate the number of moles of HCI in 62.85 mL of 0.453 M hydrochloric acid.
1) 28.5 mol
2) 1.04 mol
3) 0.139 mol
4) 0.0285 mol
5) 0.00721 mol
Answers
Answer:
Option 4
Explanation:
Hydrochloric acid is a strong one, that gives protons to medium. It can be dissociated as this:
HCl → H⁺ + Cl⁻
M means Molarity. It is a sort of concentration that indicates the moles of solute in 1L of solution.
M = moles / volume (L)
We can also say M = mmoles / mL of solution
M . mL = mmoles
0.453 M . 62.85mL = 28.5 mmoles
If we divide by 1000 → 28.5 mmol . 1 mol / 1000 mmol = 0.0285
I need these questions answered

Answers
Answer:
Three objects with kinetic energy
A ball rolling down the street
Moving Car
Bullet
Law of Conservation of Energy states that the total energy of an isolated system remains constant; it is said to be conserved over time.
Though technically there are limitless forms of both types of energy, officially there's five types of kinetic energy: radiant, thermal, sound, electrical and mechanical and potential energy adds gravitational, nuclear, and elastic.
Chemistry scavenger hunt-Can you figure it out?
1. Period two group one is where I sit
2. The # of valence electrons in the previous answer plus 23 is my atomic number
3. Five groups to the right of the previous answer, in period five, is my location.
4. the # of neutral particles in the previous answer is my atomic #
5. if you reverse the atomic number of the previous answer, you will know my mass
Answers
The chemical element in group one that is also a member of period 2 is lithium.
The chemical element in group one that is also a member of period 2 is lithium therefore, the element referred to here is lithium. Group 1 has only one valence electron. If we add one to 23, we will have 24 and the element is chromium.
For question three, the focus is on period 5, five groups to the previous answer will give us the element molybdenum. The atomic number of Mo is 42 and its mass number 96 hence it has 54 neutrons. The element that has atomic number 54 is Xe.
The element that has atomic mass 43 which is reversal of the atomic number of the former answer is scandium.
Learn more: https://brainly.com/question/967776
element that has the electron configuration of [Rn]7525f146a107p5
Answers
Answer:
Do you mean Tennessine?
Explanation:
It's in the 7th period, and has the shorthand of Rn. And it's 5 into the row.
What are the characteristics of nonvascular plants? (select all that apply) A . They don’t rely on roots to receive water.
B .They have xylem vessels and phloem vessels
C . They are able to push water and nutrients to each part of the plant.
D . They are found in moist environments.
Answers
Answer:
D . They are found in moist environments.
Explanation:
Nonvascular plants do not have a xylem or phloem, roots, stems, or leaves. Because these plants lack water-conducting tissues, they fail to achieve the structural complexity and size of most vascular plants and have evolved in habitats which allow their survival and reproduction.
The plant body that is most obvious in non-vascular plants are the the gametophyte generation. The gametophte gemeration is haploid.
The non-vascular plants grow in moist environments. It is due to lack of vascular tissue that requires to maintain close contact with water to prevent desiccation. Nonvascular plants are plants that do not have any special internal pipelines or channels to carry water and nutrients. Instead, nonvascular plants absorb water and minerals directly through their leaflike scales. Nonvascular plants are usually found growing close to the ground in damp, moist places. Non-vascular plants thrive in damp conditions since they don't need to rely on roots to acquire enough water.
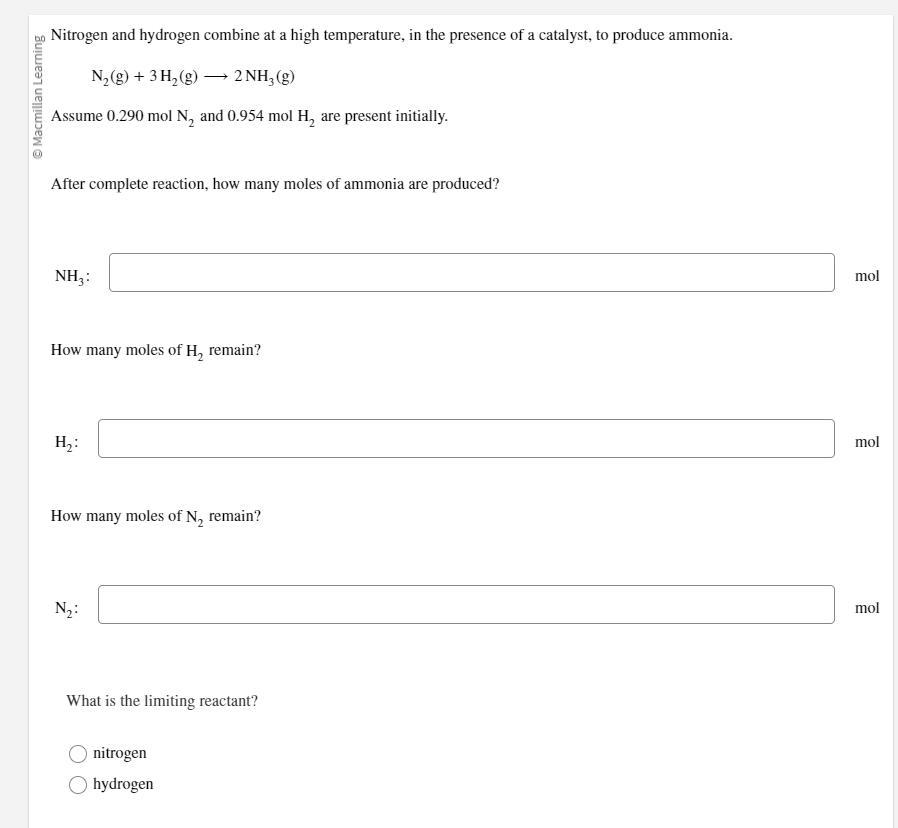
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
Answers
Answer:
17 reactions
Explanation:
1. Currently, California students are in class 240 minutes every day. How many hours is this?
Answers
Answer:
4 hours a day
Explanation:
240/60=4
have a great day :)
Explain why pure iron is malleable
Answers
Answer:
Metals are malleable : They can be bent and shaped without breaking. This is because they consist of layers of atoms that can slide over one another when the metal is bent, hammered or pressed.
Explanation:
Pure iron is a bright silvery white metal which oxidizes (rusts) rapidly in moist air or in water containing dissolved oxygen. It is soft, malleable, and ductile, and is strongly magnetic (ferromagnetic).
how many liters of water are needed to prepare 756 g of CaCO3 for a 3.50 M solution? MM=100.1 grams/mole
Answers
The volume of water needed to prepare the solution given the data is 2.16 L
What is molarity?This is defined as the mole of solute per unit litre of solution. Mathematically, it can be expressed as:
Molarity = mole / Volume
How to determine the mole of CaCO₃Mass of CaCO₃ = 756 g Molar mass of CaCO₃ = 100.1 g/mol Mole of CaCO₃ =?Mole = mass / molar mass
Mole of CaCO₃ = 756 / 100.1
Mole of CaCO₃ = 7.55 moles
How to determine the volume Mole of CaCO₃ = 7.55 molesMolarity = 3.5 MVolume = ?Volume = mole / molarity
Volume = 7.55 / 3.5
Volume = 2.16 L
Learn more about molarity:
https://brainly.com/question/15370276
#SPJ1
In order for rollercoaster to work, why does the hill the cart climbs need to be higher than the top of the loop?
Answers
Answer:
"At the top of the first and tallest hill, your potential energy is at its highest it will ever be on this ride. As you begin to descend, your potential energy decreases until it's all gone at the bottom of the hill." The shorter the hill the roller coaster climbs, the greater its kinetic energy.
Which one is it I don’t know

Answers
Using the prefix method for covalent compounds, the names of the given compounds are shown below:
LIF: Lithium fluoride
Cl2O7: Dichlorine heptoxide
N2O3: Dinitrogen trioxide
SF6: Sulfur hexafluoride
Na3PO4: Sodium phosphate
What are covalent compounds?A covalent bond is described as a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
The properties of covalent compounds includes:
The boiling/melting points of covalent compounds are low.covalent compounds are soft in nature and relatively flexible.covalent compounds do not possess electrical conductivity.Learn more about covalent compounds at:
https://brainly.com/question/3447218
#SPJ1
A compound is found to contain 3.622 % carbon and 96.38 % bromine by mass. To answer the question, enter the elements in the order presented above.
QUESTION 1: The empirical formula for this compound is .
QUESTION 2: The molecular weight for this compound is 331.6 amu. The molecular formula for this compound is
Answers
Question 1 : the empirical formula for the compound is CBr₄.
Question 2 : the molecular formula for the compound is CBr₄.
To determine the empirical formula and molecular formula of the compound, we need to analyze the given percentage composition and molecular weight. Let's go through the process step by step:
Empirical Formula:
The percentage composition by mass states that the compound contains 3.622% carbon and 96.38% bromine. We can assume a 100g sample of the compound to simplify the calculations.
Mass of carbon = (3.622/100) * 100g = 3.622g
Mass of bromine = (96.38/100) * 100g = 96.38g
Next, we need to find the moles of each element. We can use their atomic masses to convert the masses to moles.
Atomic mass of carbon (C) = 12.01 g/mol
Atomic mass of bromine (Br) = 79.90 g/mol
Moles of carbon = Mass of carbon / Atomic mass of carbon = 3.622g / 12.01 g/mol ≈ 0.3017 mol
Moles of bromine = Mass of bromine / Atomic mass of bromine = 96.38g / 79.90 g/mol ≈ 1.205 mol
To find the simplest whole-number ratio between the elements, we divide both moles by the smallest number of moles (0.3017 mol in this case):
Moles of carbon (C) = 0.3017 mol / 0.3017 mol = 1
Moles of bromine (Br) = 1.205 mol / 0.3017 mol ≈ 4
Therefore, the empirical formula for the compound is CBr₄.
Molecular Formula:
Given that the molecular weight (molar mass) of the compound is 331.6 amu, we need to compare it with the empirical formula weight.
Empirical formula weight = (Atomic mass of carbon × Number of carbon atoms) + (Atomic mass of bromine × Number of bromine atoms)
= (12.01 amu × 1) + (79.90 amu × 4) = 12.01 amu + 319.6 amu = 331.61 amu
The molecular weight is very close to the empirical formula weight, indicating that the empirical formula represents the molecular formula as well. Therefore, the molecular formula for the compound is also CBr₄.
Know more about Molecular formula here:
https://brainly.com/question/15960587
#SPJ8
In a coffee cup calorimeter, 1.60 g of NH4NO3 is mixed with 75.0 g of water at an initial temperature of 25.008C. After dissolution of the salt, the final temperature of the calorimeter contents is 23.348C. Assuming the solution has a heat capacity of 4.18 J 8C21 g21 and assuming no heat loss to the calorimeter, calculate the enthalpy change for the dissolution of NH4NO3 in units of kJ/mol.
Answers
Answer: ΔH for the dissolution of \(NH_4NO_3\) is +26.0205 kJ/mol
Explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
\(Q=m\times C\times \Delta T\)
Q = Heat released by solution = ?
C = heat capacity = \(4.18J/g^0C\)
Initial temperature of water = \(T_i\) = \(25.008^0C\)
Final temperature of water = \(T_f\) = \(23.348^0C\)
Change in temperature ,\(\Delta T=T_f-T_i=(23.348-25.008)^0C=-1.66^0C\)
Putting in the values, we get:
\(Q=75.0g\times 4.18J/g^0C\times -1.66^0C=-520.41 J\)
As heat released by water is equal to heat absorbed by dissolution of \(NH_4NO_3\)
\(\text{Moles of}NH_4NO_3=\frac{\text{given mass}}{\text{Molar Mass}}=\frac{1.60g}{80g/mol}=0.02mol\)
Enthalpy change for 0.02 moles of \(NH_4NO_3\) = 520.41 J
Enthalpy change for 1 mole of \(NH_4NO_3\) = \(\frac{520.41}{0.02}\times 1=+26020.5J=+26.0205kJ\)
ΔH for the dissolution of \(NH_4NO_3\) is +26.0205 kJ/mol
for which electrode could you use an inactive material
Answers
Mercury and carbon two electrodes can't be used with an inactive or inert material.
What is an electrode?The electrode is the element which is used to complete the electric circuit in welding. Some time electrode is connected with the positive terminal and sometimes with a negative terminal, it depends on the requirement of the welding process.
Inert electrode is an electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction. Precious metals, mercury, and carbon are typically used as inert electrodes.
Therefore, neither of the two electrodes can be used with an inactive or inert material.
Learn more about electrodes here:
https://brainly.com/question/13098144
#SPJ1
Compare the number of moles of H ions to the number of moles of OH ions in the titration mixture when the HCL is exactly neutralized by the KOH
Answers
Answer:
When HCl (hydrochloric acid) and KOH (potassium hydroxide) are neutralized, they react to form water (H2O) and a salt (KCl). The balanced equation is:
HCl + KOH → KCl + H2O
In this reaction, one mole of HCl reacts with one mole of KOH to form one mole of water and one mole of KCl.
During titration of HCl with KOH, the point at which the reaction is complete is called the equivalence point. At the equivalence point, the moles of H+ ions and OH- ions are equal in the titration mixture.
Since one mole of HCl reacts with one mole of KOH, and H+ ions are present in HCl and OH- ions are present in KOH, the number of moles of H+ ions will be equal to the number of moles of OH- ions at the equivalence point.
Therefore, at the equivalence point, the number of moles of H+ ions will be equal to the number of moles of OH- ions in the titration mixture when HCl is exactly neutralized by KOH.
When the HCl is neutralized by KOH, the equivalence point is reached. During titration, the amount of HCl is determined using a basic solution of known concentration.
It is possible to calculate the amount of KOH required for complete neutralization if the initial concentration of the HCl solution is known. The balanced chemical equation for the reaction between HCl and KOH is:HCl + KOH → KCl + H2OThe stoichiometry of the reaction indicates that one mole of HCl reacts with one mole of KOH to produce one mole of H2O. Thus, the number of moles of H+ ions is equal to the number of moles of OH- ions when the equivalence point is reached.In an acid-base reaction, the number of moles of hydrogen ions (H+) produced by the acid is equal to the number of moles of hydroxide ions (OH-) produced by the base. When the HCl is exactly neutralized by the KOH, the number of moles of H+ ions is equal to the number of moles of OH- ions in the titration mixture.
This is due to the balanced chemical equation for the reaction, which shows that one mole of HCl reacts with one mole of KOH to produce one mole of water (H2O).Thus, at the equivalence point, the number of moles of H+ ions is equal to the number of moles of OH- ions. This is the point at which all of the HCl has reacted with the KOH. After the equivalence point, the excess KOH will react with the H2O to produce OH- ions, resulting in a basic solution.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
Concentration (mol dm-³) 0.5- 0.4- 0.3- 0.2- 0.1 2. 3 5 The following equilibrium reaction is given: 2HI(g) = H₂(g) + I₂(g) Time (s) H₂/ HI Cy A change in pressure will not affect equilibrium in this case as the number of moles of gas is the same on both sides of the equation. AH> 0 A graph plotting the concentrations of the substances present versus time is given in Figure 7.10. a) b) Explain the physical situation in the container from t=0 s to t = 5 s. Which external factor was altered in order to bring about a change in the shape of the graph at t = 5 s? Explain. Calculate Kat t = 3 s. 1 dm³ COCI, decomposes
Answers
Based on the information provided, we have a reaction between hydrogen iodide (HI) gas and hydrogen gas (H₂) to form iodine gas (I₂). The equilibrium is represented by the equation:
2HI(g) = H₂(g) + I₂(g)
The concentration values given in the table correspond to the concentrations of H₂ and HI at different times.
a) From t=0 s to t=5 s: Without the specific graph mentioned in Figure 7.10, it is difficult to provide a precise explanation of the physical situation in the container during this time period. However, based on the equilibrium reaction given, we can make some general observations. At the start (t=0 s), the concentrations of H₂ and HI may be high. As time progresses, the reaction proceeds, and the concentrations of H₂ and HI may decrease while the concentration of I₂ increases. The specific behavior will depend on the rate of the forward and reverse reactions.
b) External factor altered at t=5 s: To bring about a change in the shape of the graph at t=5 s, some external factor must have been altered. The most likely factor is the total pressure within the container. Since the reaction involves gases, changes in pressure can affect the equilibrium position. However, according to the information given, a change in pressure will not affect equilibrium in this case since the number of moles of gas is the same on both sides of the equation. Therefore, if the shape of the graph changes at t=5 s, some other external factor, such as temperature or the addition of a catalyst, must have been altered.
c) Calculation of K at t=3 s: The equilibrium constant (K) can be calculated at any given time using the concentrations of the reactants and products. However, the concentrations of H₂ and HI at t=3 s are not provided in the information given. Without the necessary data, it is not possible to calculate K at t=3 s.
Lastly, the statement "1 dm³ COCI, decomposes" seems incomplete. If you provide additional information or clarify the question, I'll be happy to assist you further.
If during the investigation it is found that mineral A can scratch Mineral B, but Mineral B cannot scratch Mineral A, then which of these statements is true?
Mineral A is smoother than Mineral B
Mineral A is rougher than Mineral B
Mineral A is harder than Mineral B
Mineral A is softer than Mineral B
Answers
Answer:
Mineral A is harder than mineral B
#1 pls help me complete

Answers
Answer:
n = 0.045 mol.
Explanation:
Hello there!
In this case, according to the given problem, we can firstly set up the ideal gas equation as shown below:
\(PV=nRT\)
Thus, as we are asked to calculate the moles, we proceed as follows:
\(n=\frac{PV}{RT}\)
Therefore, we plug in the given data to obtain:
\(n=\frac{760mmHg*\frac{1atm}{760mmHg}*1.0L}{0.08206\frac{atm*L}{mol*K}*273K} \\\\n=0.045mol\)
Best regards!
Scientists are studying several genes. Breeding experiments show that 10 percent of the offspring are recombinants for genes A and B. Crosses with genes C and D produce 15 percent recombinants.
What can be said about the two pairs of genes?
Genes A and B are on the same chromosome; genes C and D are located on different chromosomes.
Genes A and B are on the same chromosome; genes C and D are located on different chromosomes.
The distance between genes A and B is greater than the distance between genes C and D.
The distance between genes A and B is greater than the distance between genes C and D.
Genes A and B are on different chromosomes; genes C and D are located on the same chromosome.
Genes A and B are on different chromosomes; genes C and D are located on the same chromosome.
The distance between genes A and B is less than the distance between genes C and D.
Answers
Between genes A and B, there is a wider separation or distance than between genes C and D.
What proportion of recombinants do we use to assess if two genes are related?The chance of recombination is 50% when the genes are distant from one another or on different chromosomes. In this instance, the two loci's allele inheritance is independent. Recombination frequency less than 50% indicates a connection between the two loci.
Which applications of genetic mapping are crucial?A illness that is passed from parent to kid may be linked to one or more genes with certainty thanks to genetic mapping. Moreover, mapping can reveal which chromosome a gene is located on and where exactly it is located on that chromosome.
To learn more about genes visit:
brainly.com/question/8832859
#SPJ1
Fill in the blanks: Electrons are ___ in an ionic bond, whereas they are___ in a polar covalent bond, and ___ in a nonpolar covalent bond.
Answers
Answer:
The correct answer is "transferred; unequally shared; equally shared".
Explanation:
Ionic bonding occurs when a positively charged atom (cation) interacts with a negatively charged atom (anion). In ionic bonding, the cation transfers its electron to the anion. In polar covalent bonding, electrons are unequally shared. This means that the electrons spend more time in an atom than the other, which gives partial positive and negative charges to the atoms. On the other hand in nonpolar covalent bonding, the electrons are equally shared and no charges are created.
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
It is a hot summer day. Your dog, Spot, is lying in the shade, panting. The water content in the dog's blood gets low. Spot gets up
to find________ because he is responding to the internal stimulus of_______
A)
food; hunger
B)
water; thirst
C)
his bed; sleepiness
D)
shade; high temperatures
Answers
What are the major species present in 0.250 M solutions of each of the following acids? Calculate the pH of each of these solutions.a.HOC6H5b. HCN
Answers
The pH of a 0.250 M solution of HOC6H5 is 2.92 and HCN is 5.96
a. HOC6H5, or benzoic acid, is a weak acid that partially dissociates in water to form H+ and C6H5COO- ions. The dissociation reaction is
HOC6H5 ⇌ H+ + C6H5COO-
To determine the pH of a 0.250 M solution of benzoic acid, we need to calculate the concentration of H+ ions in solution. The equilibrium expression for the dissociation reaction is:
Ka = [H+][C6H5COO-]/[HOC6H5]
where Ka is the acid dissociation constant for benzoic acid. The value of Ka for benzoic acid is 6.5 × \(10^-5.\)
We can assume that the initial concentration of H+ ions in the solution is zero. At equilibrium, let x be the concentration of H+ ions in solution and 0.250 - x be the concentration of C6H5COO- ions in solution. Then:
Ka = x(0.250 - x)/0.250
Solving for x, we get:
x = 1.21 × \(10^-3\) M
the pH of a 0.250 M solution of benzoic acid is:
pH = -log[H+] = -log\((1.21 × 10^-3)\) = 2.92
b. HCN, or hydrocyanic acid, is also a weak acid that partially dissociates in water to form H+ and CN- ions. The dissociation reaction is:
HCN ⇌ H+ + CN-
To determine the pH of a 0.250 M solution of HCN, we need to calculate the concentration of H+ ions in solution. The equilibrium expression for the dissociation reaction is:
Ka = [H+][CN-]/[HCN]
where Ka is the acid dissociation constant for HCN. The value of Ka for HCN is 4.9 ×\(10^-10\).
We can assume that the initial concentration of H+ ions in the solution is zero. At equilibrium, let x be the concentration of H+ ions in solution and 0.250 - x be the concentration of CN- ions in solution. Then:
Ka = x(0.250 - x)/0.25
Solving for x, we get:
x = 1.1 × \(10^-6\) M
pH :- -log[H+] = -log\((1.1 × 10^-6)\)
= 5.96
For more questions on HCN
https://brainly.com/question/10108557
#SPJ4
Give me an example of an average. For instance, "the average height of a person is about 5
foot 6 inches
Answers
Answer:
What is the average height of a human?
5.6 ft.
5.2 ft.
Human/Height
Image result for Give me an example of an average. For instance, "the average height of a person is about 5 foot 6 inches
However, the actual global average height of a woman is only 159.5 cm (5 ft 2.8 in) and the average height of a man is 171 cm (5 ft 7.3 in). Therefore, the height difference between men and women globally is about 4.5 inches or 12 centimeters.
Explanation:
what is inside an atom
Answers
For a particular reaction at 135.4
°C, Δ=−775.41 kJ/mol
, and Δ=817.91 J/(mol⋅K)
.
Calculate ΔG for this reaction at 12.7
°C.
Answers
Answer:
\(\Delta G=-675.38 \frac{kJ}{mol}\)
Explanation:
Hello!
In this case, for this problem, it is possible to use the thermodynamic definition of the Gibbs free energy:
\(\Delta G=\Delta H-T\Delta S\)
Whereas G, H and S can be assumed as constant over T; thus, we can calculate H at 135.4 °C:
\(\Delta H=\Delta G+T\Delta S\\\\\Delta H=-775.41\frac{kJ}{mol}+(135.4+273.15)K*(0.81791\frac{kJ}{mol*K} )\\\\\Delta H=-441.58\frac{kJ}{mol}\)
Now, we can calculate the Gibbs free energy at 12.7 °C as shown below:
\(\Delta G=-441.58\frac{kJ}{mol} -(12.7+273.15)K*0.81791\frac{kJ}{mol*K}\\\\\Delta G=-675.38 \frac{kJ}{mol}\)
Best regards!
What kind of graph shows how data change over time, with no lines
connecting the data points?
Answers
Answer:
Bar Graphs
Explanation:
Answer: Scatterplot
Explanation:

C3H8O2
empirical Or molecular
formula formula
Answers
Answer: i think its empirical
Explanation:
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
NEED ANSWER NOW! 40 POINTS
Read the chemical equation.
Fe2O3 + CO → Fe + CO2
if 1.8 moles of Fe2O3 react with 2.7 moles of CO, how many moles of each product are formed?
5.4 moles Fe and 1.8 moles CO2
2.7 moles Fe and 0.9 moles CO2
3.6 moles Fe and 5.4 moles CO2
1.8 moles Fe and 2.7 moles CO2
Answers
Answer:1.8 moles Fe and 2.7 moles CO2
Explanation:
Answer:
1.8 moles Fe and 2.7 moles CO2