Answers
Answer:
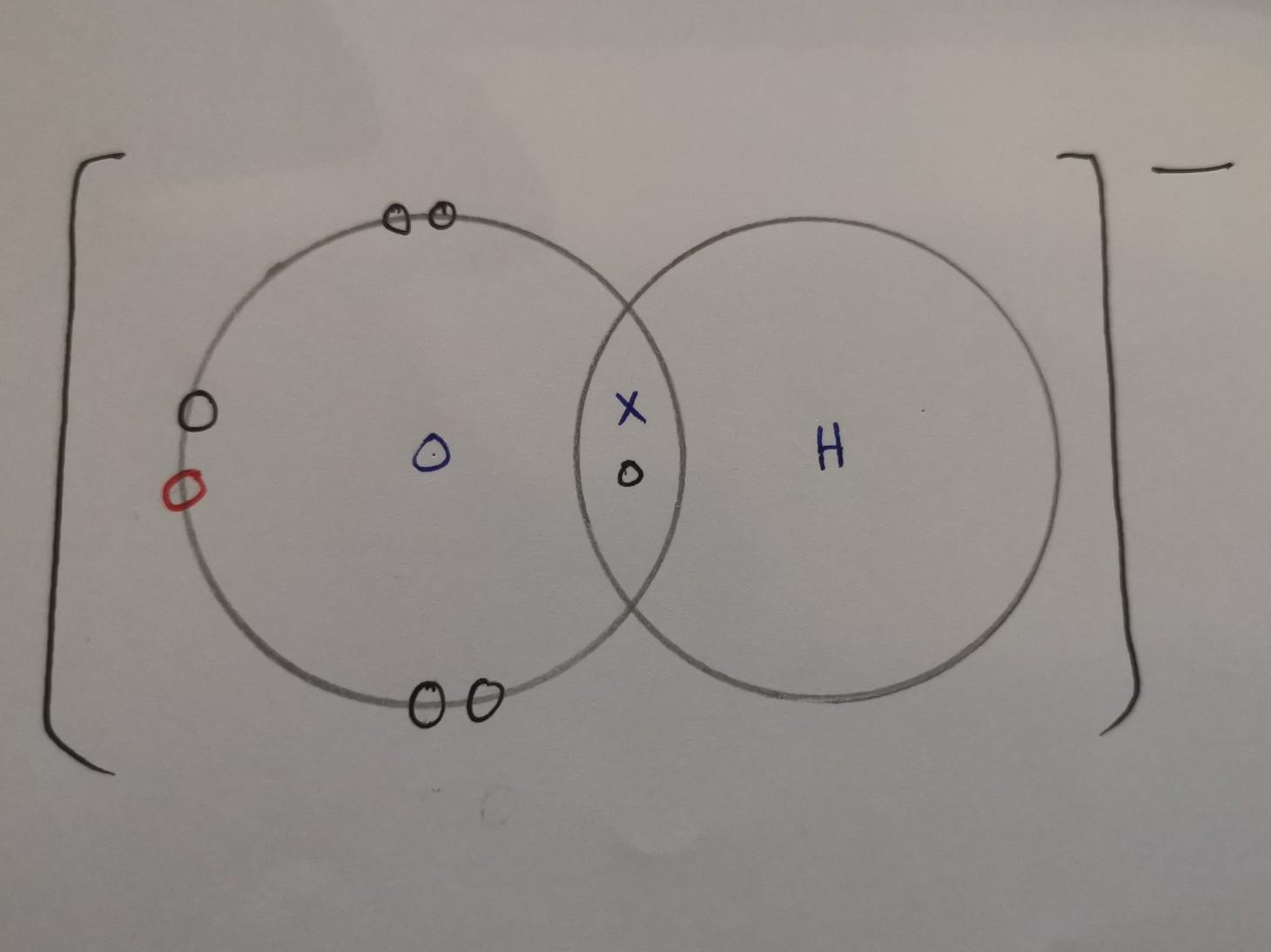
the electrons would be exchanged kn the reaction = 2e

Related Questions
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
1. Measuring instruments, such as rulers and graduated cylinders, are often used in experiments to increase the A clarity of the hypothesis B. testability of the hypothesis C. accuracy of the data sample size of the data 5
Answers
Answer:
C. accuracy of the data sample size of the data
Explanation:
Measuring instruments, such as rulers and graduated cylinders, are often used in experiments to increase the accuracy of the data sample size which is usually gotten from the taken reading.
Measuring instruments such as rulers have accurate readings which makes measurements in any form very easy and more accuracy.
6) What is the molarity of the solution that contains 0.5 moles of calcium chloride in 485 mL of solution
Answers
The molarity of the solution that contains 0.5 moles of calcium chloride in 485 mL of solution is 1.03 M.
Molarity is a measurement of concentration that refers to the amount of moles of a solute per liter of solution. In order to determine the molarity of the solution that contains 0.5 moles of calcium chloride in 485 mL of solution, the information must be converted into the correct units.
Molarity is , `Molarity= moles of solute/volume of solution in liters
`First, the volume of the solution needs to be converted from milliliters to liters:
1 L = 1000 mL; therefore, 485 mL = 0.485 L
Next, the molarity of the solution can be calculated as follows:
Molarity = 0.5 moles / 0.485 L = 1.03 M
The molarity of the solution that contains 0.5 moles of calcium chloride in 485 mL of solution is 1.03 M.
Learn more about molarity here: https://brainly.com/question/30404105
#SPJ11
If a solution has a pH of 14, would it be considered to be a stong acid, weak acid, strong base, or weak base?
Answers
Answer: The solution with pH of 14 will be a strong base.
Explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
\(pH=-log[H^+]\)
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7. Thus pH scale range from 1 to 14.
Strong acids have low pH ( around 1 ) values where as strong bases have high pH ( around 14) values.
Answer:
on my test the answer was a strong acid
Explanation:
warm-blooded animals employ ______ to regulate the temperatures of the body
a) radiation
b) conduction
c) convection
Answers
Warm-blooded animals employ radiation to regulate the temperatures of the body. Therefore, the correct option is option A.
What is warm-blooded animal?The term "warm-blooded" relates to animal species that bodies sustain a temperature higher than the ambient temperature. Homeothermic creatures (birds and mammals included) control metabolic activities to maintain a constant body temperature. The degree of thermoregulation in other animals varies.
Because animals employ more than two methods of temperature regulation, the terms "warm-blooded" and "cold-blooded" have become derogatory within the scientific community. Warm-blooded animals employ radiation to regulate the temperatures of the body.
Therefore, the correct option is option A.
To learn more about warm-blooded animal, here:
https://brainly.com/question/11278471
#SPJ9
13. An organic compound is found to contain 77.42% of C, 7.53% of H and
nitrogen. The mass of 1.12L of its vapour at NTP is 4.65g. Determine
the
empirical and molecular formula of the compound.
Answers
Answer
7.53% 97% if you divide it you can get the answer
Explanation:
a 25.0-ml sample of hcl is titrated with naoh. if 28.9 ml of 0.175 m naoh is needed to reach the endpoint, what is the concentration (m) of the hcl solution?
Answers
The concentration of the HCl solution is 0.2023 M.
The concentration (molarity) of HCl can be calculated from the given data. This calculation requires knowledge of the balanced chemical equation between HCl and NaOH. For the neutralization reaction between HCl and NaOH, the balanced chemical equation is shown below:
HCl + NaOH → NaCl + H₂O
From the balanced chemical equation above, we can see that 1 mole of HCl reacts with 1 mole of NaOH to produce 1 mole of NaCl and 1 mole of H₂O.
To calculate the concentration (molarity) of HCl, we can use the equation below:
M₁V₁ = M₂V₂
where M₁ is the concentration of the NaOH solution, V₁ is the volume of the NaOH solution used to titrate the HCl solution, M₂ is the concentration of the HCl solution, and V₂ is the volume of the HCl solution.
M₁ = 0.175 M (given) V₁ = 28.9 mL (given) M₂ = ? V₂ = 25.0 mL (given)
Substituting the given values into the equation,
we get: 0.175 M × 28.9 mL = M₂2 × 25.0 mL
We can solve for M₂ by dividing both sides of the equation by 25.0 mL:
M₂ = (0.175 M × 28.9 mL) / 25.0 mL = 0.2023M
Therefore, the concentration of the HCl solution is 0.2023 M (rounded to four significant figures).
Learn more about molarity on:
https://brainly.com/question/30404105
#SPJ11
6. A diamond contains 5.0 ×10^21 atoms of carbon. What amount(moles) of carbon and what mass (grams) of carbon are in this diamond? *
a. 0.008303 and 0.0997
b. 0.008303g and 0.0997mol
c. 0.008303amu and 0.0997
d. 0.008303 and 0.0997amu
Answers
Answer:
0.008303 mol and 0.0997 g
Explanation:
From Avogadro's hypothesis, we understood that 1 mole of any substance contains 6.022×10²³ atoms.
This implies that 1 mole of carbon also contains 6.022×10²³ atoms.
Next, we shall determine the mole of carbon that contains 5.0×10²¹ atoms. This can be obtained as follow:
1 mole of carbon contains 6.022×10²³ atoms.
Therefore, Xmol of carbon will contain 5.0×10²¹ atoms i.e
Xmol of carbon = 5.0×10²¹ / 6.022×10²³
Xmol of carbon = 0.008303 mole
Therefore, 0.008303 mole of carbon contains 5.0×10²¹ atoms.
Finally, we shall determine the mass of carbon. This can be obtained as follow:
Mole of carbon = 0.008303 mole
Molar mass of carbon = 12.01 g/mol
Mass of Carbon =.?
Mole = mass /Molar mass
0.008303 = mass of carbon/12.01
Cross multiply
Mass of Carbon = 0.008303 x 12.01
Mass of Carbon = 0.0997 g.
how do humans impact the distribution of minerals?
Answers
Answer:
With our modern tools and increased rate of mining industries being built, id say we deal a big impact on mineral exportation.
Explanation:
1. Which of the following is not an example of matter
a) smoke
b) air
c) heat
d) water vapor
Answers
Answer:
C. Heat
Explanation:
bc its right ik it
Answer:
C) Heat
Explanation:
Heat is not made up of atoms and molecules, it is a form of energy
How did water form the Grand Canyon?.
Answers
Flowing water from the Rocky Mountains formed the mighty Colorado River. As the plateau rose, rivers cut through it, carving canyons over time.
When water freezes it expands into ice. Ice widens cracks and erodes rock in the process. During short periods of heavy rain, water rolls down cracks and erodes more rocks and stones. The canyon becomes wider at the top than at the bottom as more rock is eroded away.
The Grand Canyon is perhaps the best example of a water-sculpted canyon. Water exerts tremendous erosive power, especially when carrying large amounts of sediment and rocks, as the Colorado River does during floods. The Grand Canyon is a large deep river canyon in northwest Arizona. Water was the main cause of the erosion that formed the Grand Canyon.
Learn more about The Grand Canyon here:-https://brainly.com/question/2143035
#SPJ4
How many milliliters of 0.400 M NaOH are required to completely neutralize 20.0 mL of 0.200 M HCl?
A) 50.0 mL
B) 40.0 mL
C) 0.100 mL
D) 20.0 mL
E) 10.0 mL
Answers
Answer:
10 mL
Explanation:
First we need to have the equation and make sure its balanced
NaOH + HCl -> NaCl + H2O
Since the ratio is 1:1 we can use M1V1=M2V2
So V1=M2V2/M1
(20.0mL)(0.200M)/(0.400M) = 10.0mL
Which of the following forms of invisible light, would have the highest wavelength?
Group of answer choices
you snap chatting your friends
a microwave warming up some mac-n-cheese
someone getting dental x-rays taken
a set of U/V (ultraviolet) lights sanitizing the lab safety glasses
Answers
Answer:
you snap chatting your friends
Explanation:
The waves that will have the highest wavelength from the given choices is by snap chatting with your friends.
Snap chatting involves the use of radio waves. Radio-waves are a part of electromagnetic waves and they have very long wavelength. It is used in radio communications as in mobile phones. x-rays have the shortest wavelength of all the waves.If 9000. J of heat are absorbed by 800. g of water at 25.0oC, what will be its final temperature?
Answers
Answer:
22 626 000 J. = 22 626 kJ. 4. If 9000 J of heat are absorbed by 800 g of water at 5.0 o. C, what maximum temperature will the water attain? Q = mc ∆T.
Explanation:
The final temperature can be calculated using the calorimetric equation. Here, the final temperature of water is 27.71 °C.
What is calorimetric equation ?The calorimetric equation connecting the heat energy absorbed or released q by a system with the mass m, temperature difference ΔT and the specific heat capacity c as follows:
q = m c ΔT
The specific heat capacity of water = 4.15 J/ °C g.
given that heat energy absorbed q = 9000 J
mass = 800 g
initial temperature = 25 °C.
Then,
9000 J = 4.15 J/ °C g. × 800 g (25 °C + T)
then T = [ 9000 J/ 4.15 J/ °C g. × 800 g ] + 25 °C = 27.71 °C.
Therefore, the final temperature of water is 27.71 °C.
Find more on calorimetry:
https://brainly.com/question/30884598
#SPJ2
mixed aldol reaction. what is the major reason why you would observe formation of at least one side product
Answers
The major reason why you would observe the formation of at least one side product of a mixed aldol reaction is due to the presence of multiple reactive sites on both carbonyl compounds.
The mixed aldol reaction involves the condensation of two different carbonyl compounds (such as aldehydes and ketones) in the presence of a base catalyst. The major reason why we would observe the formation of at least one side product of a mixed aldol reaction is that the presence of multiple reactive sites on both carbonyl compounds can lead to unwanted side reactions, such as self-condensation of one of the carbonyl compounds or formation of a cross-condensation product. The presence of water or other protic solvents can also contribute to the formation of side products by promoting competing reactions, such as hydrolysis or elimination. Therefore, careful control of reaction conditions and selection of appropriate reactants is important to minimize side product formation in mixed aldol reactions.
Learn more about mixed aldol reaction: https://brainly.com/question/31430038
#SPJ11
38 grams of lithium carbonate is dissolved in 183ml of solution. What is the molarity solution?
Answers
Answer
Molarity of the solution = 2.81 mol/L
Explanation
Given:
Mass of lithium carbonate = 38 grams
Volume of solution = 183 mL
What to find:
Molarity of the solution.
Step-by-step solution:
The molarity of the solution can be calculated using the molarity formula, which is;
\(Molarrity=\frac{Mole}{Volume\text{ }in\text{ }L}\)First, you need to convert 38 grams of lithium carbonate to mole using the mole formula.
Molar mass of lithium carbonate = 73.891 g/mol
\(Mole=\frac{Mass}{Molar\text{ }mass}=\frac{38\text{ }g}{73.891\text{ }g\text{/}mol}=0.51427102\text{ }mol\)Also, Volume in L = (183/1000) = 0.183 L
Putting the values of mole and volume in L into the molarity formula above, we have;
\(Molarity=\frac{0.51427102\text{ }mol}{0.183\text{ }L}=2.81\text{ }mol\text{/}L\)Hence, the molarity of the solution is 2.81 mol/L.
Which of the following is a result of the specific heat differences between land and ocean?
A. Ocean tides are created.
B. Volcanoes are created.
C. Saltwater is created.
D. Breezes are created.
Answers
expression for calculating the charge of an ion
Answers
Answer:
Number of protons - number of electrons = charge on ion.
Explanation:
Carbon cycles through various reservoirs on earth. the time it takes to form these reservoirs varies greatly. which process in the carbon cycle takes millions of years to form the indicated reservoir?
Answers
The process in the carbon cycle that takes millions of years to form the indicated reservoir is the formation of fossil fuels.
Carbon cycles through various reservoirs on earth. The time it takes to form these reservoirs varies greatly.
What exactly is fossil fuels?Fossil fuels are formed by the decomposition of organic materials that are buried under sedimentary rocks.
It is a long process that takes millions of years.
These fuels are important sources of energy for humans, but they have a negative impact on the environment.
The carbon cycle is the process in which carbon moves between various reservoirs on earth.
The reservoirs include the atmosphere, the oceans, the land, and the living organisms.
Carbon moves through these reservoirs through various processes, including photosynthesis, respiration, and decomposition.
The time it takes for carbon to move through these reservoirs varies greatly.
Some processes, such as photosynthesis and respiration, occur quickly and can be measured in seconds or minutes.
Other processes, such as the formation of fossil fuels, take millions of years to form.
Over time, carbon can become trapped in different reservoirs.
For example, carbon can become trapped in fossil fuels, which are formed from the remains of dead organisms.
These reservoirs can then be used as sources of energy, but the process of using them releases carbon into the atmosphere, which contributes to global warming.
Learn more about fossil fuels here:
https://brainly.com/question/10172005
#SPJ11
do the sp2 carbons and the indicated sp3 carbons lie in the same plane?
Answers
The sp² carbons and the indicated sp³ carbons are not lie in the same plane.
What is hybridization ?The phenomenon of hybridization is the combination of two atomic orbitals to produce a new degenerate hybrid orbital with the same energy levels. Hybridization improves bond formation stability over unhybridised orbitals. The hybridization of molecules allows us to predict their shape.
Because pi bonds prevent atoms from rotating, they will be in the same plane if they have a double or triple bond. Furthermore, if the atoms are trigonal or square planar, they are in the same plane.
Thus, The sp² carbons and the indicated sp³ carbons are not lie in the same plane.
To learn more about the hybridization, follow the link;
https://brainly.com/question/14140731
#SPJ1
Be + O2 --> BeO
Balance this and what's the type of reaction?
Answers
Answer:
2Be + O2 = 2BeO
its a synthesis
Explanation:
what is lost or gained during a nuclear reaciton
Answers
Answer:
Electrons
Explanation:
In nuclear fission, atomic nuclei split into lighter atoms through loss of protons and neutrons (such as through loos of a beta particles - 2 protons and 2 neutrons). ... Electrons are not involved in nuclear reactions. Electrons are mainly involved in chemical
In the Haber Process, ammonia is synthesized from nitrogen andhydrogen:
N2 (g) + 3H2 -----> 2NH3(g)
ΔG at 298K for this reaction is -33.3 kj/mol. the valuef ΔG at 298 K for a reaction mixture that consists of 1.9 atmN2, 1.6 atm H2 and 0.65 atm NH3 is________.
a.) -3.86 x 103
b.) -1.8
c.) -7.25 x 103
d.) -40.5
e.) -104.5
Answers
The value of ΔG at 298 K for a reaction mixture containing 1.9 atm N2, 1.6 atm H2, and 0.65 atm, the answer is (a) -3.86 × 10^3.
NH3 can be calculated using the equation:
ΔG = ΔG° + RT ln(Q)
where ΔG is the standard Gibbs free energy change, ΔG° is the standard Gibbs free energy change at standard conditions, R is the gas constant, T is the temperature in Kelvin, and Q is the reaction quotient.
In this case, we are given ΔG° as -33.3 kJ/mol. To calculate Q, we need to use the partial pressures of the gases in the reaction mixture. The reaction stoichiometry tells us that the ratio of the partial pressures of N2, H2, and NH3 is 1:3:2. Therefore, we can write:
Q = (P(NH3))^2 / (P(N2) * P(H2)^3)
Plugging in the given values of P(N2) = 1.9 atm, P(H2) = 1.6 atm, and P(NH3) = 0.65 atm, we can calculate Q. Then, using the value of R = 8.314 J/(mol·K) and the temperature T = 298 K, we can substitute these values into the equation and solve for ΔG.
The calculated value of ΔG at 298 K for the given reaction mixture is approximately -3.86 × 10^3 J/mol. This value is equivalent to -3.86 kJ/mol. Therefore, the answer is (a) -3.86 × 10^3.
To learn more about Haber Process here brainly.com/question/30928282
#SPJ11
2. Briefly list and describe radiocarbon and radiopotassium
dating methods. What chemical process forms the basis of the
method? How, in general, does each work? Time frame? (10-15
sentences explanati
Answers
Radiocarbon dating, also known as carbon-14 dating, is a method used to determine the age of organic materials. It is based on the radioactive decay of the isotope carbon-14 (14C).
Living organisms constantly absorb carbon, including a small amount of carbon-14, from the atmosphere. When an organism dies, it no longer takes in carbon-14, and the existing carbon-14 begins to decay at a known rate. By measuring the ratio of carbon-14 to stable carbon isotopes (carbon-12 and carbon-13) in a sample, scientists can estimate the time that has elapsed since the organism's death. Radiocarbon dating is effective for dating materials up to about 50,000 years old.
Therefore, both radiocarbon dating and radiopotassium dating rely on the principles of radioactive decay. The decay rates of the isotopes used in these methods are well-established and constant, allowing for accurate age determinations.
For more details regarding radiopotassium dating methods, visit:
https://brainly.com/question/32267082
#SPJ4
When a plant experiences heat, what part of a plant allows for the response to take place?
Answers
Calculate the pH of a solution prepared by mixing 50 mL of a 0.10 M solution of HF with 25 mL of a 0.20 M solution of NaF. The pKa of HF is 3.14.
A) 3.14 B) 10.80 C) 5.83 D) 7.35 E) 12.00
Answers
The pH of the solution is A) 3.14. To calculate the pH of the solution, we need to use the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to its acid dissociation constant (pKa) and the ratio of the concentrations of the acid and its conjugate base. The correct answer is option-a.
HF is the acid in this case, and NaF is its conjugate base. We know the pKa of HF is 3.14, so we can calculate the Ka as 10^-pKa, which gives us 7.9 x 10^-4.
Next, we need to determine the concentrations of HF and NaF in the mixture. We can do this by using the formula:
moles = Molarity x volume (in liters)
For HF, we have:
moles = 0.10 M x 0.050 L = 0.005 moles
For NaF, we have:
moles = 0.20 M x 0.025 L = 0.005 moles
Therefore, the total moles of the acid and its conjugate base are equal, and the ratio of their concentrations is 1:1.
Plugging in these values into the Henderson-Hasselbalch equation, we get:
pH = pKa + log([NaF]/[HF])
pH = 3.14 + log(0.005/0.005)
pH = 3.14 + 0
pH = 3.14
Therefore, the pH of the solution is A) 3.14. Therefore, the correct answer is option-a.
For more question on solution
https://brainly.com/question/25326161
#SPJ11
a student doing a chemistry experiment has a beaker that contains 128 ml (milliliters) of an alcohol and water solution. the lab directions indicate that there is 5.4 times as much water as alcohol in the solution. how many milliliters of alcohol are in the solution? how many milliliters of water are in the solution?
Answers
The Let's denote the volume of alcohol in the solution as A in milliliters and the volume of water as W in milliliters. According to the problem, there is 5.4 times as much water as alcohol in the solution, which can be written as W = 5.4 * An Also, we know that the total volume of the solution alcohol + water is 128 ml, s A + W = 128.
The Now, we can substitute the expression for W from the first equation into the second equation A + 5.4 * A = 128
Combine the terms with A 6.4 * A = 128 Now, solve for A = 128 / 6.4 A = 20 So, there are 20 milliliters of alcohol in the solution. Next, we need to find the volume of water W. We can use the first equation W = 5.4 * A W = 5.4 * 20 W = 108
Therefore, there are 108 milliliters of water in the solution. In summary, the solution contains 20 ml of alcohol and 108 ml of water.
learn more about alcohol here.
https://brainly.com/question/16975086
#SPJ11
What is the overall enthalpy change dhrxn for the system? -1,300 kj -300 kj 300 kj 1,300 kj
Answers
The overall enthalpy change i.e ΔH for the system is -1300 kJ.
Enthalpy change in a reaction is defined as the difference in the potential energy of the products and the potential energy of the reactants.
This is represented by ΔHreaction.
The reaction of enthalpy change is given as
ΔHreaction= ΔHproducts - ΔHreactants
where ΔHproducts is the potential energy of the products
=[(-200) + (-300)]kJ
= -500kJ
And, ΔHreactants is the potential energy of the reactants
=800KJ
Therefore, by putting these values ΔHreaction can be calculated as
ΔHreaction
= [-500-800] KJ
=-1300 KJ
Thus, the overall enthalpy change is -1300KJ
To know more about enthalpy change here
https://brainly.com/question/12200084
#SPJ4
what is the bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals?
Answers
The bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Bond order is defined as the number of electrons in bonding molecular orbitals minus the number of electrons in antibonding molecular orbitals divided by two. As a result, we may determine the bond order of this diatomic particle by the formula: Bond order = (number of bonding electrons - number of antibonding electrons) / 2
Bond order = (8 - 5) / 2
Bond order = 1.5.
This diatomic molecule, according to the bond order, is a stable molecule since the bond order is greater than 1, indicating that it is a double bond. The molecule has an overall bond strength that is greater than a single bond, but not as strong as a triple bond. So therefore he bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Learn more about bond order at:
https://brainly.com/question/30641030
#SPJ11
5. When 62.7 g of nitrogen and excess oxygen react they generate nitrogen dioxide. If the NO2 is collected at 620 K and 0.72 atm, what volume will it occupy?___ N2 (g) + ___O2 (g) → ___ NO2 (g)
Answers
answer and explanation
the first step is to balance the reaction equation
N₂ + 2O₂ > 2NO₂
now that we have balanced the equation we can calculate the number of moles of nitrogen
mols = mass / molar mass
= 62.7 g/ 14.00g/mol
= 4.48 mols
we see from the balanced equation that the mol ratio of nitrogen and nitrogen oxide is 1:2
therefor the number of mols of nitrogen oxide that will form will be
2 x 4.48 = 8.96 mol of nitrogen oxide.
now that we have the mols we can then calculate the volume using the ideal gas equation
PV =nRT
V = (nRT)/P
= (8.96 x 0.0821 x 620) / 0.72
= 633.5L
