Answers
Answer:
30.8 grams
Explanation:
n = 3.5 mol/L × 0.275 L = 0.9625 mol
The molar mass of CH3OH is 32 g/mol.
Therefore, the mass of CH3OH required is:
mass = n × molar mass
mass = 0.9625 mol × 32 g/mol = 30.8 g
Therefore, you need 30.8 grams of CH3OH to prepare a solution that is 3.5 M CH3OH in 275 mL of water.
Related Questions
How can Climate Change impact the formation of Hurricanes?
I will give brainlist
Answers
Are the following Endothermic or Exothermic?
1. Water begins to boil
2. Fireworks explode
3. Sweat evaporates cooling your skin
4. Water begins to freeze into ice
5. You ignite your homework
assignments once the year is over.
6.Sunrays burn your skin
7.Cellular respiration
Answers
1. Water begins to boil - Endothermic
2. Fireworks explode - Exothermic
3. Sweat evaporates cooling your skin - Endothermic
4. Water begins to freeze into ice - Exothermic
5. You ignite your homework assignments once the year is over - Exothermic
6.Sunrays burn your skin - Exothermic
7.Cellular respiration - Exothermic
What is Exothermic and Endothermic?Exothermic and endothermic refer to two types of chemical reactions or physical processes that release or absorb energy, respectively.
Exothermic reactions release energy in the form of heat, light, or sound. When these reactions occur, they produce a net release of energy into the surroundings, which can often be felt as an increase in temperature. Examples of exothermic reactions include combustion, oxidation, and many types of explosions.
Endothermic reactions, on the other hand, absorb energy from the surroundings in order to proceed. These reactions typically feel cold to the touch and require energy to be added to the system in order to occur. Examples of endothermic reactions include melting, evaporation, and many types of chemical reactions that require heat to be added in order to proceed.
Learn about Exothermic reactions here https://brainly.com/question/2924714
#SPJ1
uestion 8 Calculate the percentage by mass of hydrogen in PtCl2(NH3)2 A. 1.558 B. 1.008 c.0.672 D. 0.034 E.2.016
Answers
The percentage by mass of hydrogen can be calculated from the problem as 2.016
How do you calculate the mass percent of an atom in a compound?To calculate the mass percent of an atom in a compound, you first need to determine the molar mass of the compound and the molar mass of the atom of interest.
Determine the molar mass of the compound by adding up the atomic masses of all the atoms in the compound.
Determine the number of moles of the atom of interest in one mole of the compound. This is done by dividing the atomic mass of the atom by the molar mass of the compound.
We know that the relative molecular mas of the compound is; 300 g/mol
Then;
Percent by mass of hydrogen is; 6/300 * 100/1
= 2.016%
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
How many liters would you need to make a 1 m solution if you have 6 mol of sodium hydroxide.
Answers
The liters would we need to make the 1 M solution if we have 6 mol of sodium hydroxide of 6 L.
The moles of the sodium hydroxide = 6 mol
The molarity of the sodium hydroxide = 1 M
The expression for the molarity is as follows :
The molarity = moles / volume in L
The volume of the sodium hydroxide = moles / molarity
The volume of the sodium hydroxide = 6 / 1
The volume of the sodium hydroxide = 6 L.
Thus the volume of the sodium hydroxide is 6L in the 1 M of the solution.
To learn more about moles here
https://brainly.com/question/1097767
#SPJ4
which of the following concerning molecular geometry and dipole moments is correct? all molecules with polar bonds have a permanent dipole moment. all square planar molecules are nonpolar. all linear molecules are nonpolar. only molecules with polar bonds may have a permanent dipole moment. a molecule with nonpolar bonds could have a permanent dipole moment, depending on the molecular geometry.
Answers
Answer:
only molecules with polar bonds may have a permanent dipole moment.
Explanation:
8. There are 2850.5 miles between Houston, TX and Vancouver, Canada, site of the 2010 Olympic Games. How many
meters is that equal to if 1 mile is equal to 1.6 km? Express your answer in scientific notation
2850.5
9. A newborn baby eats & times a day At.

Answers
Answer:
4560.8km
Explanation:
2,850.5×1.6 = 4560.8km
You find the following reaction at equilibrium. What will happen if you remove some of the solid AgCl?
Ag+ (aq) + Cl- (aq) ⇌ AgCl (s)
Answers
If we remove some solid silver chloride the equilibrium will react to decrease the concentration of silver cation.
According to Le Chatelier's Principle, the system at equilibrium will react to a stress placed on the position of the equilibrium in such a way as to minimize this stress. The position of the equilibrium will shift in response to a stress placed on either the reactant or the product side.
Ag+ (aq.) + Cl- (aq.) ⇌ AgCl (s)
Equilibrium is a state of balance between opposing forces or actions that is either static or dynamic. If we remove the salt it will cause the concentration of aqueous silver cations to increase. This will place a stress on the position of the solubility equilibrium. Then the equilibrium will react in such a way as to decrease the concentration of silver cations. We have to shift to the left since the reverse reaction.
To learn more about Equilibrium please visit:
https://brainly.com/question/517289
#SPJ4
What molecules are needed to make atp
Answers
Answer:
APT is made of a nitrogen base (adenine), a ribose sugar, and three phosphate groups.
Hope this helped! :)
Answer:
The energy released from each molecule of glucose is used to attach a phosphate to each of four molecules of adenosine diphosphate (ADP) to produce two molecules of adenosine triphosphate (ATP) and an additional molecule of NADH.
Explanation:
B) Which of these is NEVER an example of freshwater? A) Pacific Ocean B) Okefenokee Swamp Mississippi River Lake Pontchartrain.
Answers
Answer:
Lake pontchartrain
Explanation:
A fire women dropped a person onto the safety net.Right before the person hit the net he had a velocity of 11.2m/s and 1800 J of kinetic energy. What was the mass of the person?
Answers
Explanation:
kinetic energy = ½mv²
1800 = ½ × m × 11.2²
1800 = ½ × m × 125.44
3600 = 125.44m
m = 3600/125.44
m = 28.7 Kg
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
6.802 x 1020 particles of Mn(NO3)3 is dissolved in 0.38 L of water.
What is the concentration of this solution in molarity?
A) 3.5 x 10-2M
B) 3.0 x 10 M
C) 0.30M
D) 0.38M

Answers
The concentration of the solution in molarity, given that 6.802×10²⁰ particles were dissolved in 0.38 L of water is 3.0×10⁻³ M (option B)
How do i determine the molarity of the solution?First, we shall determine the number of mole that contains 6.802×10²⁰ particles of Mn(NO₃)₃. Details below:
From Avogadro's hypothesis,
6.022×10²³ particles = 1 mole of Mn(NO₃)₃
Therefore, we can say that
6.802×10²⁰ particles = 6.802×10²⁰ / 6.022×10²³
6.802×10²⁰ particles = 0.001 mole of Mn(NO₃)₃
Finally, we shall obtain the molarity of the solution. Details below:
Number of mole of Mn(NO₃)₃ = 0.001 moleVolume of solution = 0.38 LMolarity of solution = ?Molarity of solution = mole / volume
Molarity of solution = 0.001 / 0.38
Molarity of solution = 3.0×10⁻³ M (option B)
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1
Which of these compounds would you expect
to contain covalent bonds? Why?
a. KCI
b. PBr3
c. CIBr
d. Nal
Answers
Hope this helps you
Why is Cobalt (Co) placed before Nickel (Ni) on the Periodic Table of the Elements even though it has a higher average atomic mass than nickel?
Nickel has fewer electrons.
Cobalt has a lower density.
Cobalt was discovered first.
Nickel has one more proton.
Answers
Answer: Nickel has one more proton.
Explanation:
The Periodic table orders elements based on their atomic number not by their atomic mass even though this order usually coincides with the atomic masses of the elements. Occasionally however, this is not the case as is proven by Cobalt and Nickel.
The atomic number of an element refers to the number of protons that the element has in its nucleus. Cobalt has 27 protons whilst Nickel has one more proton at 28 which is why it comes after Cobalt.
Answer:
lower density
Explanation:
Please help :) almost due
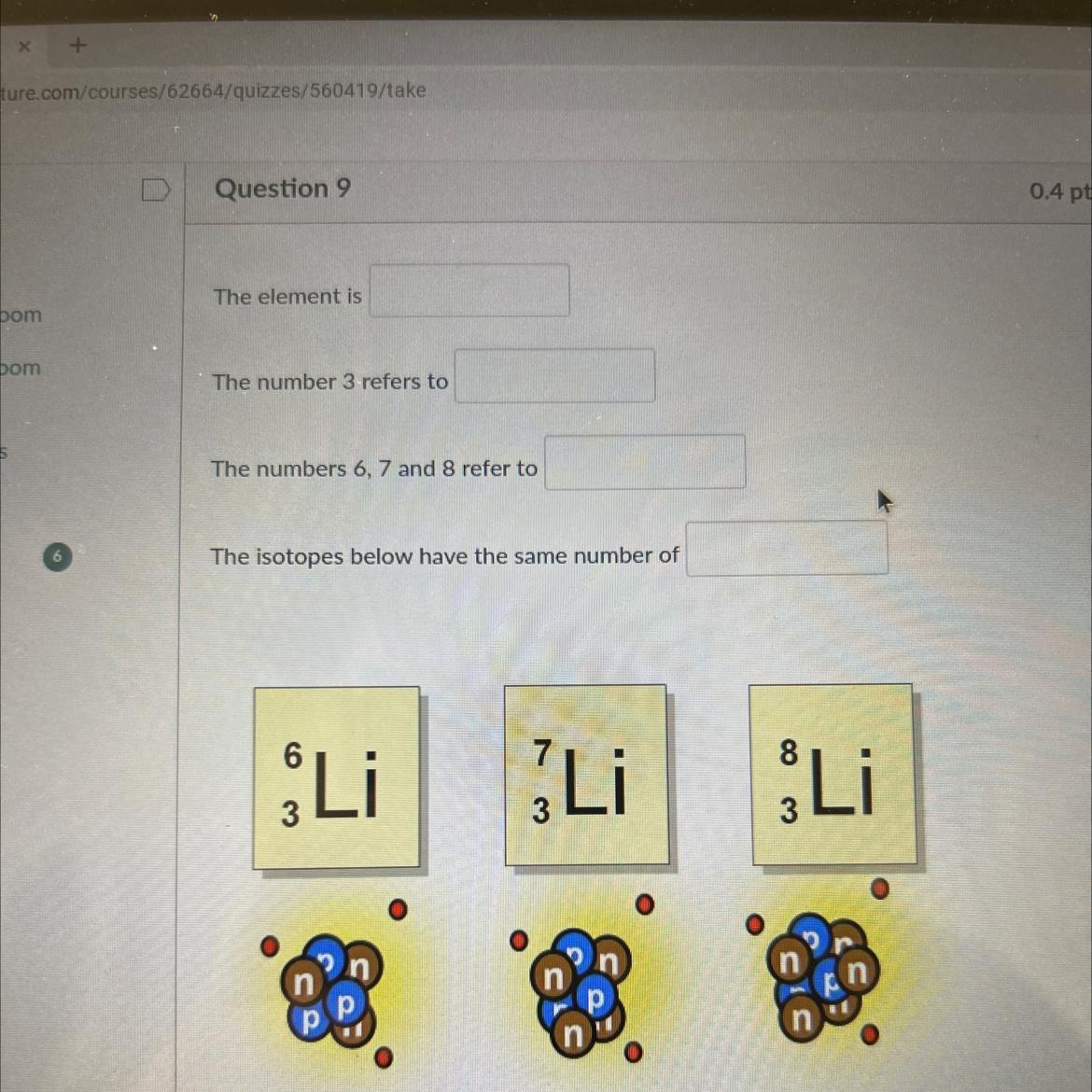
Answers
The substance is lithium (Li).
Lithium has an atomic number of 3, which corresponds to the number of protons in its nucleus.The atomic masses or mass numbers of the three isotopes of lithium are 6, 7, and 8, respectively. This indicates that the number of neutrons in the nuclei of these isotopes varies.The three isotopes of lithium, 6 Li, 7 Li, and 8 Li, all have three protons in common, but have varying numbers of neutrons in their nuclei, resulting in distinct atomic weights.Isotopes
Isotopes are atoms of the same element with variable numbers of neutrons but the same number of protons in their nucleus.
Hence, although having different atomic masses, they have the same atomic number.
The physical characteristics of an element's isotopes can differ from their chemical characteristics because of their varied atomic masses.
Isotopes include, for instance:
Carbon: There are three isotopes of carbon: carbon-12, carbon-13, and carbon-14. While Carbon-14 is a radioactive isotope, Carbon-12 and Carbon-13 are stable isotopes.There are three isotopes of hydrogen: hydrogen-1 (also known as protium), hydrogen-2 (sometimes known as deuterium), and hydrogen-3 (also known as tritium). Tritium is a radioactive isotope, whereas protium and deuterium are stable isotopes.Three oxygen isotopes are oxygen-16, oxygen-17, and oxygen-18. The most prevalent isotope is oxygen-16.learn more about isotopes here
https://brainly.com/question/29989166
#SPJ1
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
Determine the number of carbon atoms in 1.00 kg of carbon dioxide.
____X 10__atoms(Enter your answer in scientific notation.)
Determine the number of oxygen atoms in 1.00 kg of carbon dioxide.
____X 10__atoms (Enter your answer in scientific notation.)
Calculate the mass of potassium nitrate that contains 1.00 mol of oxygen atoms.
_____g
—Pls show steps!! I’m so confused and need help—
Answers
Answer:
1.37x10²⁵atoms of carbon
2.74x10²⁵ atoms of oxygen.
33.7g of KNO₃
Explanation:
To answer this question you must use molar mass of carbon dioxide (44g/mol) and 1 mole are 6.022x10²³atoms.
1.00kg are 1000g of CO₂. Moles are:
1000g CO₂ * (1mol / 44g) = 22.73 moles of CO₂ = 22.73 moles of carbon.
In atoms:
22.73 moles C * (6.022x10²³atoms / 1mole) = 1.37x10²⁵atoms of carbon
There are 22.73 moles of CO₂ * 2 = 45.45 moles of oxygen are present in the carbon dioxide. In atoms:
45.45 moles Oxygen * (6.022x10²³atoms / 1mole) = 2.74x10²⁵ atoms of oxygen.
1 mole of Potassium nitrate, KNO₃, contains 3 moles of oxygen. 1 mol of oxygen are:
1.00 mol O * (1mol KNO₃ / 3 moles O) = 0.33 moles of KNO₃
As molar mass of KNO₃ is 101.1g/mol:
0.33 moles of KNO₃ * (101.1g / mol) = 33.7g of KNO₃
Answer:
1. 1.37 * 10^25 atoms of carbon
2. 2.74 * 10^25 atoms of oxygen
3. 33.67g of Potassium Nitrate
Explanation:
2. Firstly, we want to know the number of atoms of oxygen in 1 kg of carbon dioxide.
Firstly, we will need to know the number of moles of carbon iv oxide in 1kg of carbon iv oxide
Mathematically;
number of moles = mass/molar mass
molar mass of carbon iv oxide is 44 g/mol
mass here is 1000g (1kg is 1000g)
So the number of moles of CO2 in 1kg of CO2 will be; 1000/44 = 22.73 moles
Now 1 mole of CO2 contains 2 atoms of oxygen, thus 1 mole of CO2 contains 2 moles of oxygen
So the number of moles of oxygen in 1kg CO2 will be 2 * 22.73 = 45.46 moles
Mathematically, 1 mole contains 6.02 * 10^23 atoms
So 45.46 moles will contain =
6.02 * 10^23 * 45.46 = 2.74 * 10^25 atoms of oxygen
1. 1 mole of co2 contains 1 atom of carbon , thus, 1 mole of CO2 will
contain 1 mole of carbon
From above, 1kg of carbon iv oxide contains 22.73 moles , thus 1kg of carbon iv oxide will contain 22.73 moles of carbon too
So the number of atoms will be 22.73 * 6.02 * 10^23 = 1.37 * 10^25 atoms
3. Mass of KNO3 that contains 1 mole of oxygen atom
From the formula of the compound, we can see that 1 mole of KNO3 contains 3 atoms of oxygen which translates to 3 moles of oxygen
So 1 mole of oxygen will translate to 1/3 mole of KNO3
Mathematically;
mass = number of moles * molar mass
Molar mass of KNO3 = 39 + 14 + 3(16) = 39 + 14 + 48 = 101 g/mol
So the mass will be = 1/3 * 101 = 33.67g
name the quantum number that specifies the size of an atomic orbital?
Answers
Answer:
The principal quantum number (n) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example.
1. what is an atom
2. what is a chemical process
3. WHat is a chemical reaction
Answers
Answer:
1.An atom is the defining structure of an element, which cannot be broken by any chemical means. A typical atom consists of a nucleus of positively-charged protons and electrically neutral neutrons with negatively-charged electrons orbiting this nucleus.
2.a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds.
3. a process that involves rearrangement of the molecular or ionic structure of a substance, as opposed to a change in physical form or a nuclear reaction.
What practices from the article can members of the public or governmental agencies adopt as they look to improve water quality? Back up your answer with evidence from the article above.
Answers
The evidence from the article highlights these practices as effective means of improving water quality. By implementing these measures, both individuals and governmental agencies can contribute to the preservation and protection of water resources.
According to the article, there are several practices that members of the public and governmental agencies can adopt to improve water quality:
Implementing proper wastewater management: This involves treating wastewater before it is released back into the environment. The article emphasizes the importance of implementing advanced treatment technologies to remove pollutants effectively.Promoting sustainable agriculture: The article highlights the significance of adopting practices that minimize the use of fertilizers and pesticides, such as precision agriculture techniques. These practices can reduce the runoff of agricultural chemicals into water bodies.Establishing buffer zones: Creating vegetated buffer zones along rivers, lakes, and streams can help filter and absorb pollutants, preventing them from entering the water bodies. The article suggests that buffer zones should be implemented to reduce sediment, nutrient, and pesticide runoff from adjacent fields.Encouraging responsible industrial practices: The article emphasizes the need for industries to adopt eco-friendly practices, including proper disposal of industrial waste and the use of environmentally friendly production techniques.
Raising awareness and education: Public education campaigns can play a crucial role in improving water quality. The article suggests that educating the public about the impact of their actions on water bodies and providing information on sustainable practices can lead to positive changes.
for such more questions on practices
https://brainly.com/question/30310637
#SPJ8
Which event is an example of sexual reproduction in plants?
A. Pine trees produce seeds in cones.
B. Mosses form spores in capsules.
C. A kalanchoe produces plantlets on its leaves.
D. A potato has buds that can grow into new stems.
Answers
Answer:
the correct answer to your question is b
An automobile tire is inflated to a pressure of 28.5 psi. Express this pressure in atmospheres, kilopascals, inches Hg, millimeters Hg and torr.
Hint: 1 atm = 101.3 kPa = 29.92 in Hg = 760 mm Hg = 14.69 psi = 760 torr
Answers
An automobile tire is inflated to a pressure of 28.5 psi which is equal to the 1.93930997 atm ,196.500583 kilopascals ,1473.8775 inches Hg, 1473.8 millimeters Hg, 1473.87558 torr.
or 28.5 psi = 1.93930997 atm
= 196.500583 kilopascals
= 1473.8775 inches Hg
= 1473.8 millimeters Hg
= 1473.87558 torr
What is pressure?The force delivered perpendicularly to an object's surface per unit area across which that force is dispersed is known as pressure (symbol: p or P). 445 The pressure relative to the surrounding air is known as gauge pressure, also written gage pressure.
A common mechanical quantity is pressure. Pressure is expressed using a variety of units. Some of these come from dividing a unit of force by a unit of area; the standard unit of pressure in the imperial and U.S. customary systems is the pound-force per square inch (psi), which is equivalent to one newton per square meter (N/m2) in the SI. Standard atmospheric pressure, which is equal to the atmosphere's (atm) pressure, is another way to express pressure.
Learn more about pressure: https://brainly.com/question/87231
#SPJ4
The mass fractions of a mixture of gases are 15 percent nitrogen, 5 percent helium, 60 percent methane, and 20 percent ethane with a total mixture molecular weight of 16.12 kg/kmole. Determine the mole fraction of each constituent, the partial pressure of each constituent when the mixture pressure is 1200 kPa and the apparent specific heats of the mixture when the mixture is at room temperature
Answers
Answer:
See explanation
Explanation:
Number of moles of each gas is
Nitrogen = 15/28 = 0.536 kmoles
Helium = 5/4 = 1.25 kmoles
Methane = 60/16 = 3.75 kmoles
Ethane = 20/30 = 0.67 kmoles
Total number of moles = 0.536 kmoles + 1.25 kmoles + 3.75 kmoles + 0.67 kmoles = 6.206 kmoles
Mole fraction of each gas;
Nitrogen = 0.536 kmoles/6.206 kmoles = 0.086
Helium = 1.25 kmoles/6.206 kmoles = 0.201
Methane = 3.75 kmoles/6.206 kmoles =0.604
Ethane = 0.67 kmoles/6.206 kmoles =0.108
Partial pressure of each gas;
Nitrogen = 0.086 * 1200 kPa = 103.2 kPa
Helium = 0.201 * 1200 kPa = 241.2 kPa
Methane = 0.604 * 1200 kPa = 724.8 kPa
Ethane = 0.108 * 1200 kPa = 129.6 kPa
Apparent specific heat at constant pressure;
Cp = (0.15 * 1.039) + (0.05 * 5.1926) + ( 0.6 * 2.2537) + (0.2 * 1.7662)
Cp = 2.12 KJ Kg-1 K-1
Cv = Cp- Ru/M
Cv= 2.12 - 8.314/16.12 = 1.604 KJ Kg-1 K-1
What is the energy of a purple
lamp with a frequency of
7.5 x 10^14 Hz
Answers
Answer:
\( \huge{4.969 \times {10}^{ - 19} \:J }\)
Explanation:
The energy of the purple lamp can be found by using the formula
E = hf
where
E is the energy
f is the frequency
h is the Planck's constant which is
6.626 × 10-³⁴ Js
From the question.
\( f = 7.5 \times 10^14 \: Hz \)
We have.
\( E = 6.626 \times 10^{-34} \times 7.5 \times 10^{14} \)
We have the final answer as
\(4.969 \times {10}^{ - 19} \: J\)
Chloroform is an excellent solvent for extracting caffeine from water. The distribution coefficient, KD, (Cchloroform/Cwater) for caffeine in chloroform-water at 25 oC is 10. What relative volumes of chloroform and water should be used for the extraction of 90 per cent of the caffeine from an aqueous solution in a single extraction
Answers
The relative volumes of chloroform and water that should be used is 9:10
Concentration of solution in chloroform = \(90\) ( moles of chloroform )
Concentration of solution in water = \(10\) ( moles of water )
Dissociation constant at \(25^oC\); \(K_D = 10\)
\(K_D =\) Concentration of solution in chloroform / Concentration of solution in water
Meaning;
\(K_D = \frac{\frac{mole\ of\ chloroform}{volume\ of\ chloroform} }{\frac{mole\ of\ water}{volume\ of\ water} }\)
Since \(90\) mole is present in chloroform and \(10\) mole is present in water, Total mole of Caffeine present is \(100\)
Now, we substitute our given values into the equation
\(10 = \frac{\frac{90}{volume\ of\ chloroform} }{\frac{10}{volume\ of\ water} }\\\\10 *\frac{10}{volume\ of\ water} = \frac{90}{volume\ of\ chloroform} \\\\\frac{100}{volume\ of\ water} = \frac{90}{volume\ of\ chloroform}\\\\\frac{volume\ of\ chloroform}{volume\ of\ water} = \frac{90}{100}\\\\ \frac{volume\ of\ chloroform}{volume\ of\ water} = \frac{9}{10}\\\\ \frac{volume\ of\ chloroform}{volume\ of\ water} = 9:10\)
Therefore, the relative volumes of chloroform and water that should be used is 9:10
Learn more; https://brainly.com/question/11060225
What are the charges of the ions in an ionic compound containing cobalt(III) and fluoride ions?
Write the formula for the compound.
Answers
The charge on the ions in an ionic compound containing cobalt(III) and fluoride ions is Co³⁺ and F⁻¹ and the formula of the compound is CoF₃.
Ionic compounds are a type of chemical compound where the oppositely-charged ions of a metal and a nonmetal are attracted to each other to form an ionic bond.
The compound formed from the bonded ions will have very different properties from the elements that make up the compound.
While atoms are neutral because they have an equal number of protons and electrons, ions have a net charge and result when an atom loses or gains electrons.
Learn more about Ionic compound, here:
https://brainly.com/question/3222171
#SPJ1
What is the name of the molecule shown below?
O=0
H
OH
O A. Methanoic acid
B. Methanal
C. Methanol
O D. Methylamine
Answers
Answer is
A methhanoic Acid
A group of similar or different atoms linked together makes the molecules. The molecule given is methanoic acid. Thus, option A is correct.
What is methanoic acid?Methanoic acid is a compound that has carboxylic acid as the functional group. It is the simplest carboxylic acid that has the formula HCOOH. As the molecule has a COOH group it will be acid.
The molecule can not be an aldehyde as it lacks the R−CH=O group, it cannot be alcohol as it lacks the OH group, and neither it can be methylamine as it lacks the nitrogen group.
Therefore, option A. methanoic acid is correct.
Learn more about carboxylic acid here:
https://brainly.com/question/14324837
#SPJ2
What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
1. Arrange the following groups in order of decreasing priority that would allow you to determine E/Z, or R/S. Provide a string of letters (e.g. abcd) as an answer with the highest priority listed first, lowest priority last:a) -CH3
b) -CH2OH
c) -CH2NH2
d) -CH2BR2. Arrange the following groups in order of decreasing priority that would allow you to determine E/Z, or R/S. Provide a string of letters (e.g. abcd) as an answer with the highest priority listed first, lowest priority last:a) -F
b) -CH2OH
c) -CHO
d) -CH3
Answers
Answer:
1. CH₂Br > CH₂OH > CH₂NH₂ > CH₃
2. -F > -CHO > - CH₂OH > CH₃
Explanation:
The arrangement of the above atom is due to their atomicity and electronegativity of the given compounds.
From (1) we will realize that Bromine (Br) possesses the greatest priority because its atomic number is the highest. This is followed by oxygen (O) in CH₂OH since atomic no 8 is higher than that of Nitrogen N(7). Lastly, CH₃ has the only hydrogen attached to it with the atomic no of (1)
In the second part of the question>
The electronegativity of an element increases across the period and down the group. Fluorine is highly electronegative and contains the highest atomic number of oxygen in -CHO. The oxygen (O) in -CHO has a double bond which gives an edge over the (O) in CH₂OH. Lastly, CH₃ contains a substituted hydrogen atom