How many grams of ethanol (C2H60) are required to produce 1,000
kJ during boiling? The heat of vaporization of ethanol is 38.6 kJ /
mole. Round to a whole number
Answers
16.95g is the mass of ethanol (C2H60) that are required to produce 1,000 kJ during boiling? The heat of vaporization of ethanol is 38.6 kJ / mole.
The most fundamental characteristic of matter is mass, which is one of the basic quantities in physics. Mass is a term used to describe how much matter is there in a body. The kilogramme (kg) is the international standard of mass. A nuclear reaction, for instance, results in the total weight of the substance being reduced as a tiny quantity of matter is transformed into a very large amount of energy.
moles of ethanol = 38.6/ 1,000=0.368moles
molecular weight of ethanol =46.07g/mol
mass = 0.368×46.07
= 16.95g
To know more about mass, here:
https://brainly.com/question/28704035
#SPJ1
Related Questions
Which is the best way to determine if an object is made of pure silver
Answers
Answer:
The Nitric Acid Test
Explanation:
The Nitric Acid Test is used to check if silver is pure or plated. To do so, file a small part of the item in a discreet area where it cannot be seen. Apply a few drops of nitric acid. If the area turns into creamy white, the silver is pure or sterling. If green, it is probably fake or silver-plated.
Which of the following liquids would turn
blue litmus paper red?
1. orange juice
2. milk of magnesia
3.
distilled water mixed with a little baking soda
4. oven cleaner
Answers
Answer:
orange juice because it a type of acid.
Define the term coordinate covalent bond and give an example.
please help, thank you :)
Answers
Answer:
A coordinate covalent bond is a bond in which both electrons come from the same atom.
Example: The bonding between the C atom and the O atom (carbon monoxide)
Steric strain in a large molecule is often reduced by changes in torsion angles. However, in smaller sets of fused benzene rings, like phenanthrene and 3,4-benzophenanthrene (shown below), the first geometric parameters to vary from reference values are found to be the central bond lengths and bond angles. Why do they expand to relieve steric strain before the molecule undergoes torsion to a non-planar structure?
Answers
The molecule expand to relieve steric strain before undergoing torsion to a non-planar structure in order to have several resonating structures.
What is a molecule?It should be noted that a molecule simply mean the group of atoms that are bonded together that represents the smallest fundamental unit if the chemical compound.
It should be noted that structures containing fused benzene rings have extensive conjugation. Due to this, they can undergo resonance.
This is vital in decreasing the overall energy of the molecule and helps increase its stability. When there's a change in torsion angle to relieve the steric strain, the molecule won't be planar, hence, the energy will increase and the stability will reduce.
Learn more about molecules on:
brainly.com/question/26044300
#SPJ1
19. What is the approximate mass of one mole of helium (He) gas?
A. 2 g
B. 4 g
C. 8 g
D. 22 g
Answers
Solution looked it up on google you should try it
The decomposition of HI(g) is represented by the equation
2HI(g) = H2(g) + I2(g)
The following experiment was devised to determine the equilibrium constant of the reaction.
HI (g) is introduced into five identical 400-cm3 glass bulbs, and the five bulbs are maintained at 623 K. The amount of I2 produced over time is measured by opening each bulb and titrating the contents with 0. 0150 M Na2S2O3 (aq). The reaction of I2 with the titrant is
I2 + 2Na2S2O3 = Na2S4O6 + 2NaI
Data for the experiment are provided in this table.
Bulb Initial mass of HI (g) Time(hours) Volume of titrant(mL)
1 0. 0300 2 20. 96
2 0. 0320 4 27. 90
3 0. 315 12 32. 31
4 0. 406 20 41. 50
5 0. 280 40 28. 68
What is the value of Kc for the decomposition of HI at 623 K?
Answers
The value of Kc for the decomposition of HI at 623 K is 0.0168 \(M^-^1\)
How do we calculate?\(I_2\) + \(2Na_2S_2O_3\) → \(Na_2S_4O_6\)+ 2NaI is the balanced equation:
moles of \(I_2\) = volume of titrant in mL)* (0.0150 mol/L) / 1000
for Bulb 1:
moles of \(I_2\) = (20.96 mL) * (0.0150 mol/L) / 1000
= 0.003144 mol
The concentration of \(I_2\) = moles of I2 / volume of the bulb (in L)
= 0.003144 mol / 0.400 L
= 0.00786 M
The concentration of HI = initial mass of HI / molar mass of HI / volume of the bulb (in L)
= 0.0300 g / 127.91 g/mol / 0.400 L
= 0.592 M
Kc = ([H2] * [\(I_2\)]) / ([HI]²)
Kc = [\(I_2\)] / ([HI]²)
Kc = (0.00786 M) / (0.592 M)²
Kc = 0.022 \(M^-^1\)
The Kc for each bulb
Bulb 2: Kc = 0.00834 M / (0.640 M)² = 0.020
Bulb 3: Kc = 0.00950 M / (0.788 M))² = 0.015
Bulb 4: Kc = 0.0122 M / (1.03 M))² = 0.011
Bulb 5: Kc = 0.00818 M / (0.710 M))² = 0.016
In conclusion, the average Kc
= (0.022 + 0.020 + 0.015 + 0.011 + 0.016) / 5
= 0.0168 \(M^-^1\)
Learn more about volume at:
https://brainly.com/question/27710307
#SPJ1
what is the atomic number of silver
Answers
Answer:
47
Explanation:
Silver (Ag), chemical element, a white lustrous metal valued for its decorative beauty and electrical conductivity. Silver is located in Group 11 (Ib) and Period 5 of the periodic table
The atomic number of silver is 47.
That also means that silver has 47 electrons and 47 protons.
The atomic mass of silver is 108u.
107 - 47 = 60
Silver has 60 neutrons.
What will be the pressure if the temperature is lowered to 21.663 Celsius

Answers
1.73 atm will be the pressure if the temperature is lowered to 21.663 Celsius. The correct option is C.
Thus, the coupled gas law, which states that the product of pressure and volume is exactly proportional to the absolute temperature, may be used to calculate the pressure of the gas at 21.663 degrees Celsius. If the volume stays constant, the pressure of the gas will likewise fall correspondingly as the temperature drops.
We may use the proportionality relationship to compute the final pressure using the beginning circumstances of 2.1 atm pressure, 3.78 L volume, 82°C temperature, and 21.663°C temperature. Due to the drop in temperature, the final pressure will be 1.73 atm lower than the beginning pressure.
Thus, the ideal selection is option C.
Learn more about pressure here:
https://brainly.com/question/18431008
#SPJ1
Does watermelon milk exist?
Answers
Answer:
yes
Explanation:
How many grams of AlCl3 are needed to completely react with 2.25 of NaOH?
Answers
Explanation:
hope the picture above help u understand I did it in step so it would be easier to understand:)


which of the following salts will result in basic solution in water? a. nacl b. na2s c. bacl2 d. fecl3 e. cuso4 g
Answers
In water, the Nacl salts will produce a basic solution.
The salt NaCl is hydrolyzed to produce sodium ions and hydrogen ions. Since they are both conjugate species of a strong base or acid, they do not undergo hydrolysis water and instead stay in solution as ions. In this salt solution, the pH stays constant.
A neutral salt is sodium chloride, which is produced by neutralizing sodium hydroxide and hydrochloric acid. Any strong acid that is neutralized by a strong base always produces a neutral salt.
Other salt solutions might be acidic or basic, in contrast to NaCl. One of the component ions functioning as a weak acid and weak base is the cause of this. Salt acetate is one illustration of this. The acetate ion's existence enables it to function as a diluted solution in the solution. The pH becomes basic as a result.
To learn more about salts at
https://brainly.com/question/10716016?referrer=searchResults
#SPJ4
A sample of gas has a volume of 215 cm3 at 23.5 °C and 3 atm. What will the volume of the gas be at STP
Answers
Answer:
165.3 cm^3
Explanation: hope this is correct!!
P1 * V1 / T1 = P2 * V2 / T2
P1 = 84.6 kPa
V1 = 215 cm³
T1 = 23.5°C = 23.5 + 273 K = 296.5 K
At STP:
P2 = 101.3 kPa
V2 = ?
T2 = 273 K
Draw the product for the following reaction between an alkyne and one equivalent of hcl.
Answers
To solve such this we must know the concept of addition reaction. The reaction between alkyne and HCl gives addition product. The reaction can be written as
CH\(\equiv\)CH +HCl\(\rightarrow\)CH\(_2\)=CHCl
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Alkyne is electron rich species. The reaction between alkyne and HCl gives addition product. The reaction can be written as
CH\(\equiv\)CH +HCl\(\rightarrow\)CH\(_2\)=CHCl
Therefore, the reaction can be written as
CH\(\equiv\)CH +HCl\(\rightarrow\)CH\(_2\)=CHCl
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ1
As you move from left to right across a period on the periodic table the size of an atom will
Answers
Answer:
Moving from left to right across a period, the atomic radius decreases. The nucleus of the atom gains protons moving from left to right, increasing the positive charge of the nucleus and increasing the attractive force of the nucleus upon the electrons.
Answer:the atomic radius will decrease.The nucleus of the atom will gain protons moving from left to right
Explanation:
Which of the following factors is NOT significant in determining the effectiveness of a microbial treatment?
The susceptibility of the microbe to the treatment.
The concentration of the treatment.
The toxins secreted by the microbe being treated.
The length of exposure to the treatment
Answers
Know how all of these change as temperature decreases: a) specific humidity b) relative humidity c) capacity d) dew point temperature
Answers
As temperature decreases:
a) Specific humidity: Specific humidity is the amount of water vapor in the air per unit mass of dry air. As temperature decreases, the specific humidity of the air decreases because cooler air can hold less moisture than warmer air.
b) Relative humidity: Relative humidity is the ratio of the amount of moisture in the air to the maximum amount of moisture that the air can hold at a particular temperature. As temperature decreases, relative humidity increases because the cooler air can hold less moisture, and the same amount of moisture in the air represents a higher percentage of the maximum amount of moisture the air can hold.
c) Capacity: Capacity refers to the maximum amount of moisture that the air can hold at a particular temperature. As temperature decreases, the capacity of the air to hold moisture also decreases because cooler air can hold less moisture than warmer air.
d) Dew point temperature: The dew point temperature is the temperature at which the air becomes saturated and can no longer hold all of its moisture, resulting in the formation of dew or fog. As temperature decreases, the dew point temperature also decreases because cooler air can hold less moisture than warmer air. This means that the air will reach saturation at a lower temperature, resulting in a lower dew point temperature.
To know more about temperature. here
https://brainly.com/question/26866637
#SPJ4
What’s the answer to this?
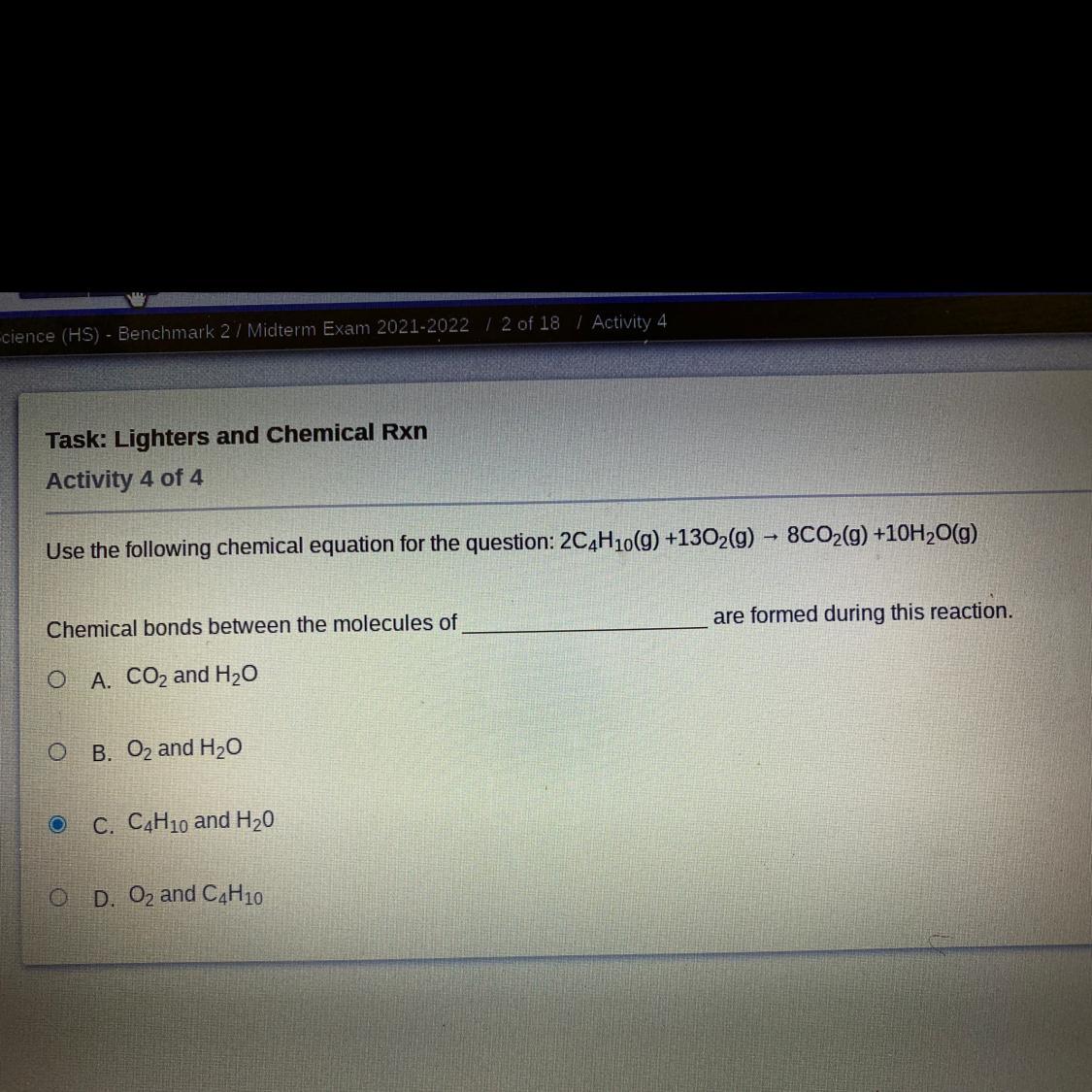
Answers
Depending on the reaction, we could monitor the progress towards equilibrium by observing __________.
Answers
Depending on the reaction, we could monitor the progress towards equilibrium by observing the concentration of the reactant and the product are equal with time.
What is equilibrium?Equilibrium is a stage of reaction in which the rate of forwarding reaction is equal to the rate of backward reaction and equilibrium is stable at the reversible state of mode.
The concentration of reactant and product must also be equal or the same as the time then only it can be an equilibrium reaction.
Therefore equilibrium depends on the reaction, the concentration of the reactant and the product are equal with time.
Learn more about equilibrium, here:
https://brainly.com/question/13463225
#SPJ4
Which of the following is NOT a reason for the experimental volume of the flask to be incorrect?
The constant temperature water bath decreases the volume occupied by the gas.
The volume labeled on the flask is not an accurate measure of the total volume of the flask.
The HCl solution added to the flask decreases the volume occupied by gas.
The rubber stopper used to seal the flask decreases the volume occupied by the gas.
Answers
Answer:
2
Explanation:
i dont andesdant
The experimental volume of the flask is found to be incorrect as the volume labeled on the flask is not an accurate measure of the total volume of the flask as it will result in errors.
What are errors?
Errors in chemical analysis result when there is a difference between observed value and the true value.If the magnitude of errors is large , it results in decrease in accuracy, reproducibility, and precision.
There are three types of errors:1) random error 2) systematic error 3) human error.The cause of random errors are difficult to quantify while the human errors can be minimized by taking a range of readings to reduce the error.
Errors while measuring boiling point may be human errors while noting down the boiling temperature or instrumental or systematic error if there is a fault in the thermometer.
Learn more about errors,here:
https://brainly.com/question/15810279
#SPJ2
enter your answer in the provided box. how many grams of koh are present in 37.5 ml of a 7.31 m koh solution?
Answers
In a 37.5 mL solution with a molarity of 7.31 M KOH, the approximate mass of KOH is calculated to be 10.91 grams, utilizing the formula involving volume, molarity, and molar mass of KOH.
The number of grams of KOH present in a solution, we need to use the formula:
grams of KOH = (volume of solution in liters) x (molarity of KOH) x (molar mass of KOH)
First, we need to convert the given volume of 37.5 mL to liters by dividing it by 1000:
volume of solution = 37.5 mL ÷ 1000 = 0.0375 L
Next, we can substitute the given molarity of 7.31 M and the molar mass of KOH (39.10 g/mol) into the formula:
grams of KOH = (0.0375 L) x (7.31 mol/L) x (39.10 g/mol)
Calculating this expression, we find:
grams of KOH = 10.91475 g
Therefore, approximately 10.91 grams of KOH are present in 37.5 mL of a 7.31 M KOH solution.
It's important to note that when working with significant figures, the final answer should be rounded to the appropriate number of significant figures based on the given data.
To know more about molarity refer here:
https://brainly.com/question/13386686#
#SPJ11
Please help, and answer as much as you can.

Answers
Answer:
∴ Fractional distillation is the technique used to separate the fraction
Explanation:
A: Refinery gas
B: Gasoline fraction
C: Naphtha
D: Kerosene
E: Diesel Oil
F: Fuel oil fraction
G: Lubricating fraction
H: Bitumen
In the procedure you are asked to measure the boiling point of your water bath, why should you not assume the boiling point is 100°C?
Answers
It is important to measure the boiling point of your water bath because water boils at different temperatures depending on altitude and atmospheric pressure. At sea level, the boiling point of water is typically 100°C (212°F), however, this temperature can vary due to changes in altitude and atmospheric pressure.
The boiling point of water is not always 100°C because it is affected by atmospheric pressure. At higher altitudes, the atmospheric pressure is lower, which causes water to boil at a lower temperature. Therefore, if you are conducting an experiment at a higher altitude, you should not assume that the boiling point of water is 100°C. Instead, you should measure the boiling point of your water bath to ensure that your experiment is accurate.
Additionally, impurities in the water can also affect the boiling point, causing it to be higher or lower than 100°C. Therefore, it is important to measure the boiling point of your water bath rather than assuming it is 100°C.
Learn more about boiling point at https://brainly.com/question/40140
#SPJ11
Write the atomic symbols for nitrogen-14 and nitrogen-15
Answers
Answer:
hope this helps
Explanation:
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Fourteen radioisotopes are also known, with atomic masses ranging from 10 to 25, along with one nuclear isomer, 11mN.
H3N
Nitrogen-15 | H3N
Which of the following could be used to sterilize objects such as medical devices?
a. ethylene oxide
b. silver nitrate
c. 100% alcohol
d. orthophenylphenol
Answers
The following could be used to sterilize objects such as medical devices:a. Ethylene oxide. Ethylene oxide (EtO) is an industrial chemical compound used in sterilization and fumigation.
It is commonly utilized to sterilize medical devices and surgical instruments that cannot be sterilized with conventional steam sterilization techniques. The ethylene oxide procedure, often known as EtO sterilization, involves exposing products to a certain concentration of ethylene oxide gas in a low-pressure chamber for a specified amount of time to achieve sterilization.
Ethylene oxide is an excellent sterilization option for items that may be damaged by heat or moisture, including medical implants, plastic containers, and packaging materials, as well as electronic instruments.The other options, such as silver nitrate, 100% alcohol, and orthophenylphenol are not typically used for the purpose of sterilizing medical devices and equipment. While silver nitrate is used in some medical applications, such as treating eye infections in newborns, it is not typically used as a sterilizing agent. Similarly, 100% alcohol is a disinfectant and can be used to clean surfaces, but it is not effective at sterilizing medical equipment.
To know more about fumigation visit:
https://brainly.com/question/29065657
#SPJ11
What is the maximum numbers of orbitals in the p sublevel
Answers
Answer:
3
Explanation:
hope the picture helps you to understand :)

Which of the following elements has only one electron in its outermost s orbital?
A. F
B. Al
C.Mg
D.Na
Answers
Answer:
D: Na
Explanation:
Hope this helps!
Have a nice day!!
:D
Mohs Scale of Mineral Hardness Hardness Mineral Absolute Hardness 1 Talc 1 2 Gypsum 2 3 Calcite 9 4 Fluorite 21 5 Apatite 48 7 Quartz 100 8 Topaz 200 According to the Moh's Scale of Mineral Hardness, the sample mineral that will scratch gypsum but not apatite is MOST LIKELY A) calcite. B) quartz. C) talc. D) topaz.
Answers
Answer: A) calcite
Explanation:
Hardness can be defined as the ability of the mineral to resist the scratch. It is the property to judge the hardness of the mineral. Mineralogist study this property of hardness using Mohs hardness scale.
Talc is the least hard substance after that gypsum, then calcite, fluorite, and then apatite.
The gypsum can be scratched by the calcite but the calcite cannot scratch apatite as it is softer than apatite.
This chart lists four kinds of polymers and their sources.
A 2-column table titled polymer examples. The first column labeled name of polymer has entries D N A, cellulose, nylon, hair. The second column titled source of polymer has entries inside of cells, plant material, synthetic material, animal material.
What can be known about all four polymers, despite their differences?
They come from living things.
They share ionic carbon bonds.
They are at least 100 monomers long.
They are made of repeating subunits.
Answers
Answer:
D. They are made of repeating subunits.
Explanation:
The first column represting polymers such as DNA, cellulose, nylon, hair are labelled with cells, plant material, synthetic material, animal material and They are made of repeating subunits.
Not all the entries in the first column come from living things as nylon comes from synthetic material (man-made). But all four (DNA, cellulose, nylon, hair) are conists of repeating unit.
DNA made up of nucleotides, cellulose is made up of rpeating units of monomer glucose, nylone is made of repeating unit of two monomers hexamethylene diamine and adipic acid, and hair is made of repeating unit of protein called keratin.
Hence, the correct answer is "D. They are made of repeating subunits."
Answer:
They are made of repeating subunits....(D)
Explanation:
hope this helps. also the person above me is correct
At a certain temperature, the concentration of H3O in pure water is 4.0 x 10-8, what is the value of the pKw of water at this temperature
Answers
The pKw of water at this temprature would be 4, by using the formula for pKw
What is pKw ?Although this dissociation is mild, water can split into its ions. The Kw is the dissociation constant, and the ionic product tends to remain constant.
Kw is the equilibrium constant that is connected to the molarity of water in an aqueous solution, so to speak. The concentration of a solute in a solution is indicated by its molarity or molar concentration, which is a volume that varies on temperature.
As a result, whereas the molarity in aqueous solutions is essentially constant, the pKw varies with temperature. When the temperature rises, the pKw decreases; when the temperature falls, the pKw rises.
To view more questions on concentrations, refer to:
https://brainly.com/question/13226191
#SPJ4
Explain the similarities and differences between putting a beaker of ethanoic acid in the refrigerator and mixing it with sodium carbonate
Answers
Answer:
See explanation
Explanation:
When a beaker of ethanoic acid is placed in the refrigerator, its temperature drops and the vessel feels cool.
Now, when we mix ethanoic acid and sodium carbonate, an endothermic reaction occurs, fizzing is observed as carbon dioxide is given off and heat is lost to the surroundings causing the reaction vessel to feel cool to touch.
The difference between putting ethanoic acid in the refrigerator and adding sodium carbonate to the solution is that, in the former, no new substance is formed. The substance remains ethanoic acid when retrieved from the refrigerator. In the later case, new substances are formed. The substance is no more ethanoic acid because a chemical reaction has taken place.