How many grams of KNO3 are needed to make 1. 50 liters of a 0. 50 M KNO3 solution?
Answers
75.83 grams of KNO3 are required to prepare a 0.50 M solution in 1.50 L of water.
To prepare a 0.50 M solution of KNO3 in 1.50 L of water, we can determine the amount of KNO3 required by using the formula:
Molarity (M) = moles of solute / liters of solution
Rearranging the formula, we can calculate the number of moles of KNO3:
moles of KNO3 = Molarity x liters of solution
Given the values, we find:
moles of KNO3 = 0.50 M x 1.50 L = 0.75 moles
To find the mass of KNO3 needed, we need to use its molar mass:
molar mass of KNO3 = 101.10 g/mol
Therefore, the mass of KNO3 required is:
mass of KNO3 = moles of KNO3 x molar mass of KNO3
Substituting the values, we obtain:
mass of KNO3 = 0.75 moles x 101.10 g/mol = 75.83 g
Hence, to prepare a 0.50 M solution in 1.50 L of water, you would need 75.83 grams of KNO3.
Know more about Molarity here:
https://brainly.com/question/22997914
#SPJ11
Related Questions
A student leaves a 10.0 g ice cube in a sealed flask on the lab table. After some time, the ice melts. What is the mass of the liquid?Less than 10.0 g10.0 gNot enough informationGreater than 10.0 g
Answers
Step 1 - Understanding the law of mass conservation
In physics, chemistry and biology the law of mass conservation states that no atom is created or destroyed, they are only rearranged in any given physical/chemical/biological process.
Therefore, if all atoms are preserved, the total mass of reactants and products is the same.
Step 2 - Answering the exercise
The melting of an ice cube is a physical process and, as such, is also subjected to the law of mass conservation. Therefore, if 10g of ice melt, 10g of water are produced.
Answer: 10.0g
How does a mixed inhibitor that has a higher affinity for the enzyme compared to the substrate affect the Michaelis-Menten constant (Km) in enzyme kinetics?
Answers
A mixed inhibitor that has a higher affinity for the enzyme compared to the substrate can affect the Michaelis-Menten constant (Km) in enzyme kinetics by increasing the value of Km.
Km is a measure of the substrate concentration required for half-maximal velocity and is a key parameter in describing enzyme catalysis. However, In the presence of a mixed inhibitor, the inhibitor molecule can bind to both the free enzyme and the enzyme-substrate complex with different affinities.
The binding of the inhibitor to the free enzyme reduces the number of active enzyme molecules available for catalysis while binding to the enzyme-substrate complex slows down the catalytic reaction. This leads to a reduction in the effective concentration of the enzyme-substrate complex, which in turn increases the apparent Km value.
In other words, the higher affinity of the mixed inhibitor for the enzyme reduces the efficiency of the enzyme-substrate complex formation, making it more difficult for the substrate to bind to the enzyme, hence increasing the value of Km. Therefore, a mixed inhibitor that has a higher affinity for the enzyme compared to the substrate can cause a decrease in the efficiency of enzyme catalysis, which is reflected in the increase in Km.
Know more about Mixed inhibitor here :
https://brainly.com/question/31590577
#SPJ11
What about 50 g of water?
I need help what this
Answers
Answer:
3.38 Tablespoons
10.14 Teaspoons
0.21 U.S. Cups
0.18 Imperial Cups
0.20 Metric Cups
50.00 Milliliters
Explanation:
All ions and isotopes of an atom share the same____
A)atomic number
B)mass number
C)radio waves
D)charge
Answers
Answer:
A atomic number
Explanation:
not too sure
What are the chemical properties of iron meta
Answers
Answer:
Physical properties
Atomic mass: 55.845
Density: 7.87 g/cm3
Group: Transition metals
Electrons: [Ar] 3d6 4s2
Melting point: 1811 K
Boiling point: 3134 K
Discovered: Ancient times
Explanation:
hope this helps! :D
The reaction between solid aluminum and liquid bromine is exothermic. This reaction creates
the product aluminum bromide.
What equation includes the correct formulas and demonstrates conservation of mass?
A Al(s) + Br2(1) - AlBr3(s)
B Al(s) + Br(l) - AlBr(s)
C Al(s) + 3Br(1) - AlBr3(s)
D
2Al(s) + 3Br2(1) - 2AlBr3(s)
Answers
Answer:
correct answer is d)
Explanation:
This answer demonstrates law of conservation of mass because the mass of the products is equal to the mass of reactants with the help of the coefficients which is the process known as balancing. This answer also includes the correct formulas, as Br is a gas so it MUST be written as Br2. hope this helps:)
2Al(s) + 3Br2(1) - 2AlBr3(s) is the correct formulas and demonstrates conservation of mass.
What is Conservation of Mass?Every action has an equal and opposite response, states Newton's Third Law. This indicates that any force applied to an item will have an equal and opposite-directed response force of the same magnitude. A force pair is the collective name for these connected forces.
Each individual object will have a different force pair with each other object in systems of numerous objects where all things interact. In the case of charged particles, the charge of each object will dictate the direction of each force in the force pair.
Similar charges will cause particles to repel one another by directing their forces away from one another. With their forces pointing in the same direction, particles with opposing charges attract one another.
Therefore, 2Al(s) + 3Br2(1) - 2AlBr3(s) is the correct formulas and demonstrates conservation of mass.
To learn more about mass, refer to the link:
https://brainly.com/question/19694949
#SPJ2
What is technology?
A. The steps that engineers go through to create a product.
B. An understanding of something new.
C. Something created using science for use by society.
D. A method that is used to solve problems.
SUBMIT
Answers
can sb helppppppppppppp

Answers
Explanation:
Copper does not react with HCl to give H2 gas.
Which of the following ground-state electron configurations represents the element with the lowest
electronegativity?
Choose 1 answer:
A [Ar]3d¹04s²4p4
B [Ar]3d¹04s²4p5
[Kr]4d²25s²
D [Kr]4d¹05825p4

Answers
The answer is A [Ar]3d¹04s²4p4. This electron configuration represents the element Argon, which has the lowest electronegativity of the elements listed. Electronegativity is a measure of an atom's ability to attract electrons, so Argon has the lowest ability to do so.
What is electron configuration?Electron configuration is an arrangement of electrons in an atom or molecule in which each orbital is occupied by the maximum number of electrons with opposite spins. It describes the way that electrons are distributed among the available orbitals of an atom. Electron configuration is important because it determines the chemical properties of the atom, including reactivity and the way it bonds with other atoms. The distribution of electrons in an atom or molecule is determined by the quantum numbers associated with each electron. These quantum numbers indicate the energy, angular momentum, and other properties of the electrons. The electron configuration of an atom or molecule is determined by solving the Schrödinger equation for that particular atom or molecule.
To learn more about electron configuration
https://brainly.com/question/26084288
#SPJ1
A electrolytic cell is set up as was done in this experiment but with a different metal. An average current of 144.2 mA is delivered for 16 minutes and 39 seconds. The cathode gains 0.1427 g in mass. If there are two moles of electrons transferred per mole of the metal, what is the molar mass of the metal?
Answers
The molar mass of the metal is 191.1g/mol.
Solution:
Since the formula for faraday's law of electrolysis is given by
Since m = 0.1427g, I = 144.2mA = 144.2mA *(1A/1000mA) = 0.1442 A= 0.1442C/s, t = 16min39s = 16min*(60s/1min) = 960+39 = 999 second Z = 2e-mol
Now put all values in the above formula
0.1427 g = (0.1442 C*999s*M*1 mole e-*1 mol/96485C*s*2e-mol)
M = (0.1427g*96485*2/0.1442*999*1)
M = 191.1538 g/mol = 191.1g/mol
Therefore the molar mass of metal will be 191.1g/mol.
For chemical elements that do not have isolated molecules such as carbon and metals, the molar mass is instead calculated by dividing by the number of atomic moles. Molar mass is defined as the mass in grams of one mole of a substance. The unit of molar mass is gram/mole, abbreviated g/mol.
Learn more about The molar mass here:- https://brainly.com/question/837939
#SPJ4
Help me pls!
What is heliuim?
Answers
Answer:
it's a chemical Element
Explanation:
symbol He
atomic number 2.
Classify the following reactions as synthesis, decomposition, single replacement or double
replacement.
1. KCIO,
2. HCI + NaOH -
3. Mg + 2HCI
4. H₂ + O₂
5.
6.
Al + NiBr₂
KCI + O₂
Al + 30₂
4
1
NaCl + H₂O
MgCl₂ + H₂
H₂O
AlBr, + 3NI
Al₂O,
systhesis
decomposition
systhesis
decomposition
Move faster with Chro
systhesis
decomposition
systhesis
decomposition
systhesis
decomposition
systhesis
decomposition
ace
single replacement
double replacement
single replacement
double replacement
single replacement
double replacement
single replacement
double replacement
single replacement
double replacement
single replacement
double replacement
Answers
We can classify the given reaction as Synthesis Reactions are : Ni + Cl₂ → NiCl₂, C₃H₆ + Br₂ → C₃H₆Br₂. Decompositions Reactions are : CaCO₃ → CaO + CO₂,2NaCl → 2Na + Cl₂, 2HI → H₂ + I₂. Single Displacement Reactions are : 2Fe + 6HCl → 2FeCl₃ + 3H₂. Double Displacement Reactions are : CaCO₃ +2HCl → CaCl₂ +H₂CO₃
What is Synthesis Reactions?In this type of reaction , two or more chemical components combine in order to form a complex product which is given as:
A + B → AB
What is Decomposition Reaction?A kind of chemical reaction which only one type of reactant results to produce of two or more than two products.
What is Single Displacement reaction?A single displacement reaction which has an another name as single replacement reaction is a type of oxidation-reduction chemical reaction in which an ion or element moves out of compound.
What is Double Displacement Reactions ?A double displacement reaction is a type of chemical reaction which tells that the reactant ions exchange its's places in order to form new products.
Thus, From the above Conclusion we can say that,
Synthesis Reactions are : Ni + Cl₂ → NiCl₂
C₃H₆ + Br₂ → C₃H₆Br₂
Decompositions Reactions are : CaCO₃ → CaO + CO₂
2NaCl → 2Na + Cl₂
2HI → H₂ + I₂
Single Displacement Reactions are : 2Fe + 6HCl → 2FeCl₃ + 3H₂
Double Displacement Reactions are : CaCO₃ +2HCl → CaCl₂ +H₂CO₃
Learn more about reactions here : https://brainly.com/question/4352413
#SPJ9
Note: The question is incomplete in the portal. Here is the complete question.
Question: Classify the following reactions as synthesis, decomposition, single replacement or double replacement.
Ni + Cl₂ → NiCl₂C₃H₆ + Br₂ → C₃H₆Br₂ CaCO₃ → CaO + CO₂ 2NaCl → 2Na + Cl₂2HI → H₂ + I₂2Fe + 6HCl → 2FeCl₃ + 3H₂CaCO₃ +2HCl → CaCl₂ +H₂CO₃2. How many moles of hydrogen are produced from the reaction of 3.86 moles of zinc with an excess of
hydrochloric acid?
Answers
Answer:
as per given balanced equation one mole Zn produces 1mole Hydrogen
when reacted with excess acid.
So 3 moles Zn will give 3moles Hydrogen
Explanation:
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
What is mole ratio ?Mole ratio refers to the ratio of the number of moles of one substance in a chemical reaction to the number of moles of another substance in the same reaction.
It is derived from the balanced chemical equation for the reaction, which indicates the relative amounts of each substance involved in the reaction.
Mole ratio is important in stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction.
The given chemical reaction is written as follows:
Zn + 2 HCl ⇒ ZnCl₂ + H₂
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
Find more on mole ratio :
https://brainly.com/question/15288923
#SPJ2
Which of these statements describes a physical property of hydrogen? Group of answer choices it is found in acids. it is less dense than oxygen gas. it reacts with oxygen to form water. it is highly flammable.
Answers
Answer:
it is less dense than oxygen gas.
Explanation:
Hydrogen is the simplest chemical element that exists. The symbol for the chemical element Hydrogen is "H" and it is a colourless, tasteless, odorless, and highly flammable gas.
Hydrogen is a chemical element found in group (1) of the periodic table and as such it has one (1) electrons in its outermost shell. Therefore, Hydrogen has an atomic number of one (1) and a single valence electrons because it has only one proton and one electron in its nucleus.
In Chemistry, the properties of a chemical element that can be observed and measured without changing its chemical nature is known as a physical property. It includes density, color, freezing point, opacity, smell, melting point, viscosity, etc.
Hence, the statement which describes a physical property of hydrogen is that it is less dense (density) than oxygen gas.
Question 6 of 10
What does it mean when a reaction is spontaneous?
O
A. The reaction requires added energy.
B. The reaction goes to completion.
C. The reaction occurs rapidly.
O O
D. The reaction happens by itself.
SUBMIT
Answers
Answer:
D
Explanation:
Does not require energy ...may be slow or fast....
Kim dissolves 70.13 g of solid sodium chloride (NaCl) in enough distilled water to make 400 mL of stock solution. What volumes of stock solution and distilled water (DI) are needed to make a 150 mL solution of 1.2 M NaCl?
Answers
This problem is providing us with the mass of solid sodium chloride and the volume of water it was dissolved in. Thus, after diluting it, the volume of the stock solution was required and found to be 60. mL according to:
Diluted solutionsIn chemistry, when we are given a stock solution with specified volume and concentration, one is able to dilute it in order to use it for a specific purpose. This, by holding the number of moles constant, one can write:
\(C_1V_1=C_2V_2\)
Where the subscript 1 stands for the stock solution and 2 for the diluted one. Thus, one first calculate the initial concentration with the mass and volume:
\(M=\frac{70.13g/(58.44 g/mol)}{0.400L}=3.00M\)
Next, we solve for the volume of the stock solution, V1, as follows:
\(V_1=\frac{C_2V_2}{C_1}\)
Finally, we plug in the given data to obtain the result:
\(V_1=\frac{1.2M*150mL}{3.00M}\\ \\V_1=60.mL\)
Learn more about diluted solutions: https://brainly.com/question/26005640
What is the meaning of this painting

Answers
Answer: things arnent always as they seem
Explanation:
019 10.0 points Which response identifies a possible y for the last-filled electron in a magnesium ION? 1. 42,0,0 2. 42,0,–1 3. 43,0,1 4. 43,3,0 5. 3,1,1 6. 42,1,1
Answers
To determine the quantum numbers of the last electron in the magnesium ion (Mg2+), we need to first determine the electronic configuration of the ion.
What are the quantum numbers of the last electron in the magnesium ion?The question is incomplete but I have to look at the electron configuration of the magnesium ion.
Magnesium (Mg) has an electronic configuration of 1s2 2s2 2p6 3s2, which means it has two electrons in the 3s orbital. When magnesium loses two electrons to form the Mg2+ ion, these electrons are removed from the 3s orbital, leaving a filled 1s orbital, filled 2s orbital, filled 2p orbital, and empty 3s orbital.
The quantum numbers of the last electron in the Mg2+ ion can be determined as follows:
Principal quantum number (n): The last electron is in the 2p orbital, so its principal quantum number is n=1.
Azimuthal quantum number (l): The azimuthal quantum number specifies the shape of the orbital and is given by the formula l = n-1. Therefore, the last electron has an azimuthal quantum number of l=1
Magnetic quantum number (m): The magnetic quantum number specifies the orientation of the orbital in space and can take integer values from -l to +l. Therefore, the last electron in the Mg2+ ion has a magnetic quantum number of m=-1, 0 or 1, since there is only one electron in the 2p orbital and it is spherically symmetric.
Learn more about quantum numbers:https://brainly.com/question/16977590
#SPJ1
Suppose that you add 26.7 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f Kf of 5.12 oC/m. With the added solute, you find that there is a freezing point depression of 3.89 oC compared to pure benzene. What is the molar mass (in g/mol) of the unknown compound
Answers
From the calculation, the molar mass of the solution is 141 g/mol.
What is the molar mass?We know that;
ΔT = K m i
K = the freezing constant
m = molality of the solution
i = the Van't Hoft factor
The molality of the solution is obtained from;
m = ΔT/K i
m = 3.89/5.12 * 1
m = 0.76 m
Now;
0.76 = 26.7 /MM/0.250
0.76 = 26.7 /0.250MM
0.76 * 0.250MM = 26.7
MM= 26.7/0.76 * 0.250
MM = 141 g/mol
Learn more about molar mass:https://brainly.com/question/12127540?
#SPJ12
a metal crystallizes with a face-centered cubic lattice. the edge of the unit cell is 442 pm. what is the diameter (in pm) of the metal atom?
Answers
The diameter (in pm) of the metal atom is 312.54 pm
Atoms are organized in a cube to form a face-centered cubic unit cell structure, with six extra complete atoms placed in the center of each cube face and a fraction of an atom at each of the cube's four corners. The atoms in the cube's corner are shared by eight more unit cells.
We must apply the equation for a face-centered cubic laticce to respond to this question:
Edge length = 8 R
where R is the atom's radius.
Replacing:
442pm = √8 R
The atom's radius is R = 156.27pm.
diameter = 2 radii for as.
The metal atom's diameter is:
156.2 x 2 = 312.54 pm
Learn more about metallic radius (r) here;
brainly.com/question/14885097
#SPJ4
Help! Please:(
Given the following chemical equation, how many grams of water are created when 50.0 grams of oxygen react with excess hydrogen ?
2H2 + O2 - -> 2H20

Answers
Answer:
About 56.3 grams of H2O
Explanation:

the atmospheric pressure on a mountain is 550 mmHg and 1 atm is equal to 760 mmHg. what is the pressure in atm? give your answer to 2 significant figures.
___ atm
Answers
Answer: 0.72
Explanation:
550 mmHg x 1 atm/ 760 mmHg = 0.72 atm
define the term inertia
Answers
Answer:
Explanation:
Enertia is an integral part of Newton's first law of motion.
It is the tendency of an object to stay at rest or to continue moving until and unless any external unbalanced force, (like, applied force or force of tension or frictional force ) is applied to either move it from rest or change its speed(in other words, accelerate it!!).
Example below, is of ball at rest (fig1) and if this ball is moving straight on a frictionless surface(like ice) it will keep moving!! until, we push it or pull it.

Can anyone help me with this question??
No links please!
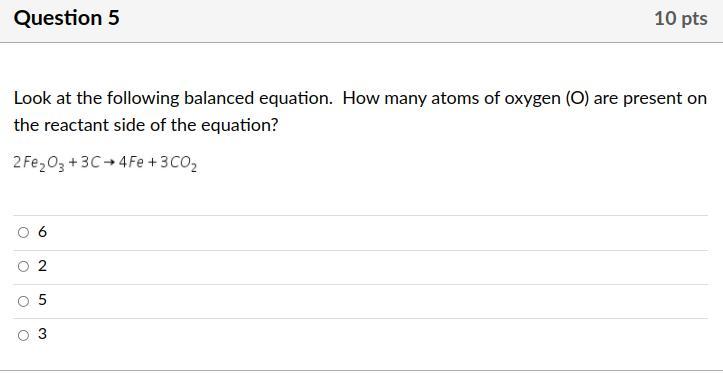
Answers
Answer: 6
Explanation: 2*3=6
Energy (eV)
Energy (EV)
Transition
nl to n2
ni to n3
nl to n4
nl to n5
nl to n6
n2 to n3
n2 to n4
n2 to n5
Transition
n2 to n6
n3 to n4
n3 to n5
n3 to n6
n4 to n5
n4 to n6
n5 to n6
Answers
Answer:
sorry i have no answer know
determine the acceptable range of mass when you are asked to obtain 3.000g of NaCl. what does this range represent?
Answers
Answer:
Explanation:
The acceptable range of mass when you are asked to obtain 3.000g of NaCl would typically be within a certain margin of error, usually +/- 0.001g or 0.1%. This range represents the precision of the measurement. It is an indication of the degree of accuracy with which the measurement was made.
When requested to obtain 3.000g of NaCl, the acceptable range of mass would normally be 0.001g. The accuracy of the measurement is represented by this range.
This range denotes the measurement's accuracy, which is a gauge of how easily the findings may be repeated. The greatest variation from the goal mass of 3.000g that is deemed acceptable is 0.001g. The measurement is more accurate the lower the deviation. Depending on the goal of the experiment, the precision of the balance utilised, and the method's sensitivity, this precision need could change. A accuracy of 0.001g is often regarded as satisfactory for the majority of laboratory applications. When working with extremely reactive or dangerous compounds or for more important applications, an accuracy of 0.0001g or less can be necessary.
learn more about mass here:
https://brainly.com/question/19694949
#SPJ4
What type of stress results when two plates converge? Compression Shear Hot spot
Answers
Answer:
Compression
Explanation:
Hi! When two convergent plates collide, they should create compressive pressure, or compression.
What happens to that atom of magnesium-24 if it GAINS a PROTON
Answers
Answer:
Adding or removing protons from the nucleus changes the charge of the nucleus and changes that atom's atomic number. So, adding or removing protons from the nucleus changes what element that atom is! For example, adding a proton to the nucleus of an atom of hydrogen creates an atom of helium
Explanation:
help please I don’t understand and this is due tomorrow.

Answers
Answer:
9.2% is the answer
Explanation:
In this question asking for the percent error of the students calculated density. The formula that will be given;
density = 9.78g/cm^3
d = 9.78g/cm^3
accepted is on the RT table S = 8.96g/cm^3
%error = (measured - accepted)/accepted * 100%
%error = (9.78 - 8.96/8.96) * 100%
%error = 9.15% or approximately 9.2%
Someone please help me!!

Answers
Answer:
the 3rd one (0.01 cm the one selected already)
Explanation:
copper wire isn't excessively big, and it wraps around the pencil because its malleable. I think that the most accurate would be 0.01 cm