How many Hydrogen (H) atoms are in the Chemical Formula(C2H2O2)2?
Answers
Answer:
4
Explanation:
in (C2H2O2)2 there are 4 hydrogen atoms
Related Questions
plz search rhe chemistry project on any topic..
Answers
IF NOT THEN SORRY :)

if 30.0 ml of 0.300 m cacl2 are added to an aqeous solution having .800g of sodium carbonante will this be enough reactant to
Answers
To determine whether there is enough reactant to completely react with the other, we need to calculate the number of moles of each substance and compare them based on their stoichiometric ratio in the balanced chemical equation. The balanced chemical equation for the reaction between calcium chloride (CaCl2) and sodium carbonate (Na2CO3) is,CaCl2 + Na2CO3 → CaCO3 + 2 NaCl
From the equation, we can see that 1 mole of CaCl2 reacts with 1 mole of Na2CO3. Therefore, we need to calculate the number of moles of each substance and compare them. Number of moles of CaCl2 moles of CaCl2 = (0.300 mol/L) x (0.0300 L) = 0.00900 mol Number of moles of Na2CO3 moles of Na2CO3 = (0.800 g) / (106.0 g/mol) = 0.00755 mol Since the stoichiometric ratio of CaCl2 to Na2CO3 is 1:1, we can see that there is less CaCl2 than Na2CO3 in the solution. Therefore, the CaCl2 will be the limiting reactant and there will be some excess Na2CO3 remaining after the reaction is complete. To determine the amount of excess Na2CO3, we can use the number of moles of Na2CO3 calculated above and subtract it from the theoretical amount of Na2CO3 that would react with all of the CaCl2, moles of Na2CO3 reacted = 0.00900 mol moles of Na2CO3 excess = 0.00900 mol - 0.00755 mol = 0.00145 mol The mass of the excess Na2CO3 can be calculated by multiplying the number of moles by the molar mass, the mass of Na2CO3 excess = 0.00145 mol x 106.0 g/mol = 0.154 g Therefore, there will be approximately 0.154 g of excess Na2CO3 remaining after the reaction is complete.
if 30.0 ml of 0.300 m cacl2 are added to an aqeous solution having .800g of sodium carbonante will this be enough reactant. In order to precipitate all of the carbonate ions from an aqueous solution of sodium carbonate, the calcium chloride solution that is added must be the excess reactant.
To know more about stoichiometric ratio click here:
brainly.com/question/30554015
#SPJ11
Did you have any difficulties building the circuit? If so, explain.
Answers
A circuit for an electronic device is made up of discrete electronic devices like resistors, transistors, capacitors, inductors, and diodes that are connected by conductive wires or traces that allow electric current to pass through them.
Electric Circuit Types
Close the loop. Closed Circuit or Closed Circuit is the term for a load that operates independently within a circuit as opposed to Open Circuit. A circuit is said to be open when an electrical line, electronic device, or switch is malfunctioning or when the switch is off. Circuit types include series, parallel, and short circuits.
There are seven causes of circuit failure: poor connections or no connections. A: You omitted a cable or connector; incorrect connection;
Noise in the circuit or close by; a poor power supply; excessive heating; incorrect design assumptions; bad components; and more.
To know more about difficulties building the circuit, click on the link below:
https://brainly.com/question/15767094
#SPJ1
Answer:
There was a problem when two wires were connected to the same part of the bulb. The batteries caught on fire in the simulation.
Explanation:
This is 100% Correct
Which processes contribute to the formation of chemical sedimentary rocks?
Answers
Answer: the answer is B: Minerals dissolve and crystallize! :D
Explanation: hope this helps!
convert 2.8 x10^27 molecules of N2O5 to grams
Answers
Answer:
2.41 molecules
Have a good day
1. The elements at the bottom of the table were pulled out to keep the table from
becoming too long. The first period at the bottom called the
Answers
Answer: Lanthanoids
Explanation:
2. Heat is:
A. thermal energy
I B. infrared radiation
C kinetic energy
D. molecular movement.
Answers
What is composed of two different atoms?
Answers
Hope I was able to help! Mark me brainly it would help a lot!:)
An object has an actual mass of 34.85 grams. Johnny measures the object to have a mass of 35 grams after rounding. What is Johnny's percent error of the object?
Answers
Answer:
1.15.........................
A container of a mixture of 4 gases has a total pressure of 35.7 kPa. Gas A has a partial pressure of 7.8kPa. Gas B has a partial pressure of 3.7 kPa, and Gas C has a pressure of 8.7kPa. What is the partial pressure of Gas D in kPa?
Answers
Answer:
partial pressure of gas D Pd = 15.5 kPa
Explanation:
As per the Dalton's law of partial pressure, in a mixture, pressure exerted by each gas when summed gives the total partial pressure exerted by mixture.
P(Total) = P1+P2+P3.....
Given P(Total) = 35.7 kPa
Partial pressure of gas A Pa = 7.8 kPa
Partial pressure of gas B Pb = 3.7 kPa
Partial pressure of gas C Pc = 8.7 kPa
There, Partial pressure of gas D Pd = P(Total) -(Pa+Pb+Pc)
Pd = 35.7-(7.8+3.7+8.7) = 35.7-20.2 kPa = 15.5 kPa
Therefore, partial pressure of gas D Pd = 15.5 kPa
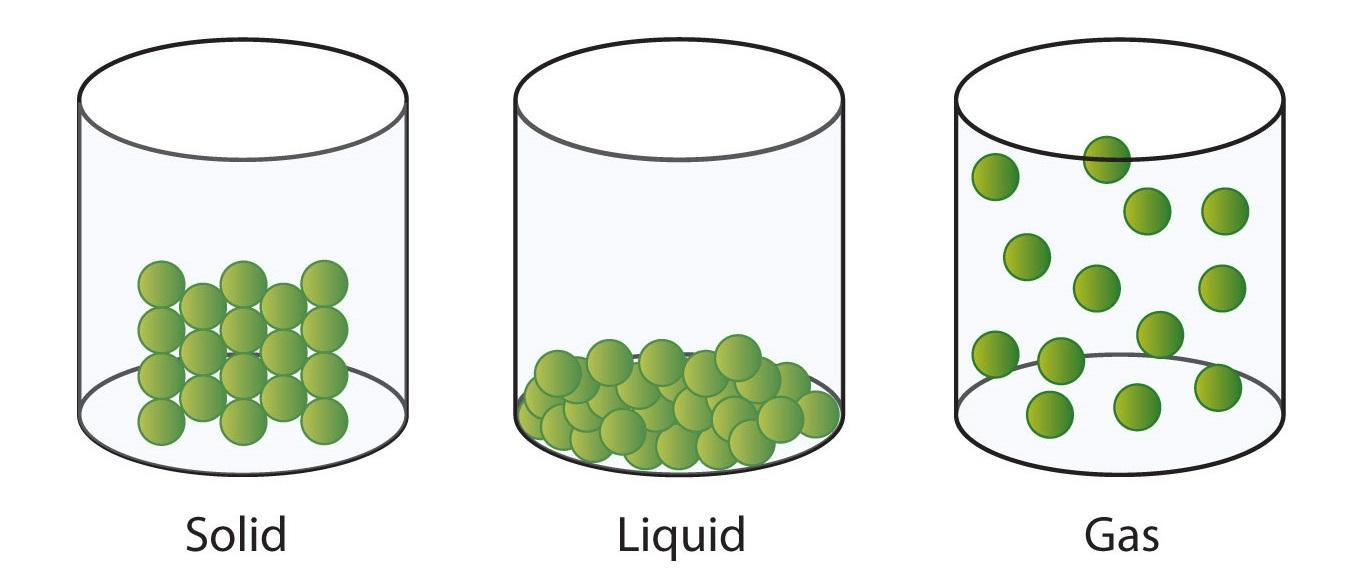
Use the particle theory to explain why 10 mL
of liquid cannot
fill a 20 mL container.
Answers
Answer:
The particle theory is the belief that everything in our solar system and beyond is made of very small matter called atoms. The 10 mL is a matter in our solar system and even though we cannot see, there are millions- if not billions of smaller particles that make up the liquid. If you view the picture below, you can see that the particles in a liquid are close to one another, particles in a gas are far apart, and a particle in a solid is tightly pushed together. This gives them their distinctive shape. Since this is a liquid, this means that the particles are close together, but not very close. The particles glide over one another. If you want to have more space between the particles and expand the size of the liquid you can boil the water, however boiling the water turns it into gas and causing the liquid to evaporate. Freezing the liquid would cause the particles to be closer packed together, making a solid. The amount of liquid you have can not change. There is still 10 mL even when the liquid is frozen or when the liquid boils into vapor in the air. Therefore using particle theory, we can know that a shape can only expand or shrink when changing states of matter.
I hope this helped & Good Luck <3 !!
HELP PLS (IT IS NOT THE THIRD ONE)

Answers
Answer:
A. would be your answer
Explanation:
Decomposers are especially important in retaining nutrients in their cells thus preventing loss of those nutrients from the root zone.
In an ecosystem, the decomposing web is important because decomposers help remove waste and use that energy to provide food for other animals.
What is an ecosystem?An ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the ecosystem through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass which is present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/13979184
#SPJ7
cyclopentane c5h10 is an alkane with a ring structure, that reacts with chlorine, cl2, to produce c2h9cl and hcl. following is a representation of a proposed mechanism for the reaction. Cl2-->2CL (Slow) Cl+C5H10-->HCl+C5H9 (Fast) C5H9+Cl-->C5H9Cl (Fast). (a0 Write the overall equation for the reaction. (b)Write a rate for the reaction that is consistent with the mechanism. Justify your answer (c) A student claims that cl2 is a catalyst in the reaction. Explain why the students claim is false.
Answers
A. We are aware that the reaction's rate depends on the slowest, or rate-determining, step in the overall reaction mechanism. The first step of the reaction is the one that moves the most slowly in the example reaction mechanism. As a result, just one step (Step 1) would have an impact on the reaction's rate.
As a result, the rate law for the mechanism described is given by –
Step 1. Cl2 2Cl (slow) ---------k1
Step 2. Cl + C5H10HCl + C5H9 (fast) ---------k2
Step 2. C5H9 + Cl C5H9Cl (fast) ---------k3
Step 1. is the slowest step. So, the reaction rate will depend upon on the rate of Step 1 only.
Hence, the rate law for the reaction is given by –
Rate = k1 [Cl2]
B. A species that is created and then consumed in situation is referred to as an intermediate of a reaction mechanism; hence, reaction intermediates never appear in the final rate law or in the actual reaction itself. The overall reaction in this reaction will show up, and likewise will be included in the rate law, as demonstrated above.
To learn more about Rate Law Reaction:
https://brainly.com/question/8139015
#SPJ1
Sabiendo el número atómico del titanio y además que su número másico es 48, ¿cuál es la cantidad de protones, neutrones y electrones respectivamente? *
Answers
Answer:
# protones: 22
# neutrones: 26
# electrones: 22
Explanation:
El número atómico del titanio es 22. Este número atómico representa la cantidad de protones del átomo en cuestión. Así:
# protones: 22.
El número másico se define como la suma de protones y neutrones, así:
48 = 22 + # neutrones
26 = # neutrones
Un átomo es neutro, esto quiere decir que sus cargas positivas y negativas son iguales (Protones: cargas positivas; Electrones: Cargas negativas).
Así, el # protones = # electrones = 22
what is weather how to fold clothes
Answers
Answer:
Weather is : the state of the air and atmosphere at a particular time and place : the temperature and other outside conditions (such as rain, cloudiness, etc.) at a particular time and place. And how to fold clothes:
Fold in one side of the shirt towards the centre, about a third.
Fold the sleeve back the other direction, away from the centre.
Long sleeves can be folded again down towards the hem.
Repeat on the other side.
Fold the collar end towards the bottom hem, making a rectangle.
Explanation:
Answer:
Weather is the state of the air and atmosphere at a particular time and place: the temperature and other outside conditions (such as rain, cloudiness, etc.) in a specific time and place. And how to fold clothes
Explanation:
Fold about a third in one side of the shirt towards the center.
Fold the sleeve back in the other direction, away from the center.
Long sleeves can be folded again down towards the hem.
Repeat on the other side.
Fold the collar end towards the bottom hem, making a rectangle.
As Earth's population continues to grow, so will the competition for our water supply. Describe three actions that our government could take to reduce competition for water use.
Answers
Answer:
Recycling waste water, awareness about efficient use of water and rain water harvesting.
Explanation:
Our government could take to reduce competition for water use by recycling waste water, awareness about efficient use of water and rain water harvesting are the methods which can reduce competition for water supply. Awareness in people about water bring great change and the people used water efficiently. Water should be harvested through making of reservoirs which collects water from rain which can be used for drinking as well as washing purpose. Recycling of waste water also increases the amount of water and reduce competition.
Are plants and animal pH sensitive?
Answers
Answer:
Plants and animals are pH sensitive. The growth of plants is dependent on the nature of the soil. If the pH of the soil is greater than 7 and is alkaline, then the plants cannot grow in the soil.
Explanation:
1. Write a hypothesis based on observations and scientific principles. (Hint: This means you write a prediction about how you think natural selection will lead to changes in the specific traits in populations of moths in the simulation. Don't forget to write it in the if-then statement format
Answers
How will you show that carbon dioxide is havier than air?
Answers
Answer:
There's an experiment that you can try to see if carbon dixide is heavier than air.
Explanation:
At room temperature, carbon dioxide has higher density (ρ = 1.98 kg·m -3) than air (ρ = 1.29 kg·m -3). The density of gas, however, significantly depends on its temperature. Carbon dioxide produced by burning has higher temperature than the surrounding air.
Why is CO2 heavier than the air?
Short answer is CO2 is heavier than ambient air, but it does not drift to the ground as there is a lot of mixing going on in ambient air. ... Carbon dioxide, also known by the chemical formula CO2, has a higher density than the other gases found in air, which makes CO2 heavier than the air.
Arrange the elements in each of the following groups
in increasing order of the most positive electron affinity: (a) Li, Na, K; (b) F, Cl, Br, I; (c) O, Si, P, Ca, Ba
Answers
The elements arranged in increasing order of the most positive electron affinity for each group are:
(a) Li, Na, K
(b) I, Br, Cl, F
(c) Ba, Ca, Si, P, O
(a) Li, Na, K: In this group, the electron affinity increases as we move from left to right in the periodic table. Therefore, the elements arranged in increasing order of the most positive electron affinity are Li, Na, and K.
(b) F, Cl, Br, I: In this group, the electron affinity generally increases as we move from left to right and from bottom to top in the periodic table. Therefore, the elements arranged in increasing order of the most positive electron affinity are I, Br, Cl, and F.
(c) O, Si, P, Ca, Ba: In this group, the electron affinity generally increases as we move from left to right and from top to bottom in the periodic table. Therefore, the elements arranged in increasing order of the most positive electron affinity are Ba, Ca, Si, P, and O.
Know more about Electron affinity here:
https://brainly.com/question/30888367
#SPJ8
When a liquid freezes into a solid, the particles of the substances *
lose energy
gain energy
move faster
disappear
Answers
Answer:
Lose energy
Explanation:
The molecules are moving from a liquid (molecules slide past one another) to a solid (molecules barely move) so are losing energy
How are reflecting telescopes different from refracting telescopes?
Answers
Reflecting telescopes are preferred for scientific research because they are better suited for gathering large amounts of light and producing high-quality images. However, refracting telescopes are still popular for amateur astronomers and for viewing objects on Earth.
Reflecting telescopes are different from refracting telescopes because reflecting telescopes use mirrors, whereas refracting telescopes use lenses. The reflecting telescope was invented in 1668 by Sir Isaac Newton, and it has since become one of the most popular types of telescopes.
Reflecting telescopes use a mirror to gather and focus light, while refracting telescopes use a lens to do the same thing. Reflecting telescopes can be made much larger than refracting telescopes because it is easier to make large mirrors than it is to make large lenses. The mirror in a reflecting telescope is placed at the back of the telescope, and it gathers and reflects light back to a secondary mirror, which then reflects the light to the eyepiece. The eyepiece is where the observer looks through the telescope.In contrast, the lens in a refracting telescope is placed at the front of the telescope, and it gathers and bends light as it passes through. The lens focuses the light onto an eyepiece at the back of the telescope. Refracting telescopes are generally smaller than reflecting telescopes because of the difficulty of making large lenses.
Another difference between reflecting and refracting telescopes is the way they are constructed. Reflecting telescopes have a simple tube that houses the mirrors and eyepiece, while refracting telescopes have a more complex design with a long tube that contains the lens and eyepiece.
for such more questions on telescopes
https://brainly.com/question/28359353
#SPJ8
at a birthday party a child sits on a partially filled balloon, decreasing its volume by 1/2. did the pressure of the ballon increase or decrease? by what factor did the pressure change?
Answers
When the child sits on the partially filled balloon, the pressure of the balloon increases. The pressure change is a doubling of the initial pressure, indicating a factor of 2 increase.
When the child sits on a partially filled balloon, the volume of the balloon decreases by half. According to Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume at constant temperature, the pressure inside the balloon increases.
Let's consider the initial volume of the balloon as V and the initial pressure as P. When the volume decreases by half, it becomes V/2. Since the amount of gas remains constant, the pressure increases to maintain equilibrium. The new pressure can be denoted as P'.
According to Boyle's Law, P₁V₁ = P₂V₂, where P₁ and V₁ are the initial pressure and volume, and P₂ and V₂ are the final pressure and volume. Plugging in the values, we have P * V = P' * (V/2).
Simplifying the equation, we get P' = 2P. This means the pressure of the balloon increases by a factor of 2, or it doubles. So, the pressure change can be expressed as an increase of two times the original pressure.
For more question pressure
https://brainly.com/question/30235826
#SPJ8
collectiveness leads to a good society? how
Answers
Answer:
A good society has a transparent talk, they share all matters related to the society, hence everyone knows what's going on. This is why collectiveness leads to a good society.
If my answer helped, kindly mark me as the brainliest!!
Thank You!!
which of the following salts produces a basic solution in water: naf, kcl, nh4cl? choose all that apply.
Answers
The salt that produces a basic solution in water is NaF.
When a salt dissolves in water, it can either produce an acidic, basic, or neutral solution, depending on the nature of the salt. Salts that are derived from strong bases and weak acids are basic solution, while salts that are derived from weak bases and strong acids are acidic in nature. Salts that are derived from either strong bases and strong acids or weak bases and weak acids are neutral in nature.
In this case, NaF is derived from the strong base NaOH and the weak acid HF, so it is a basic solution. KCl is derived from the strong base KOH and the strong acid HCl, so it is neutral in nature. NH4Cl is derived from the weak base NH3 and the strong acid HCl, so it is acidic in nature. Therefore, the salt that produces a basic solution in water is NaF.
To learn more about salt, Click here: brainly.com/question/30105881
#SPJ11
A catalyst increases the rate of reaction but is not consumed by the reaction. Catalysts lower the ____________ energy.
Answers
A catalyst increases the rate of reaction but is not consumed by the reaction. Catalysts lower the Positive catalyst energy.
Describe the catalyst.A catalyst is a substance that expedites a chemical process without being consumed by it; as a result, a catalyst can be chemically recovered unaltered at the conclusion of the reaction it has been used to catalyze.
What do Catalysts Do?With reduced activation energy and a distinct transition state, catalysts enable an alternative method for the reactants to become products. A catalyst might lower the reaction temperature, speed up the process, or improve the reaction's selectivity. Frequently, when reactants and catalysts combine, intermediates are created that ultimately produce the same reaction products and allow the catalyst to be renewed. The catalyst may be used up during one of the intermediate processes, but it will be regenerated before the reaction is finished.
Learn more about activation energy here:-
https://brainly.com/question/10507976
#SPJ4
An ion has 9 protons, 10 neutrons, and 10 electrons. The symbol for the ion is:
17O2+
17O2-
19F-
17Ne2+
Answers
Answer:
flourine 19F-
Explanation:
because we have 1 extra electron so the charge should be negative, then check the number of protons to know the atomic number, and 9 is flourine
Which transition of an electron in the hydrogen atom emits a photon with the smallest amount of energy?.
Answers
E3 is least transition of an electron in the hydrogen atom emits a photon with the smallest amount of energy.
A hydrogen atom is an atom belonging to the element hydrogen in chemistry. One positively charged proton and one negatively charged electron are both present in the electrically neutral atom and are held to the nucleus by the Coulomb force. Normal circumstances are infrequent for lone neutral hydrogen atoms. Hydrogen atoms can also exist in cationic and anionic states, however neutral hydrogen is frequently seen when it is covalently joined to another atom.
Energy is the quantifiable attribute that is transferred to a body or a physical system in physics. It is observable in the production of work as well as in the form of heat and light. The law of conservation of energy asserts that although energy can be changed in form, it cannot be created or destroyed. Energy is a conserved quantity.
learn more hydrogen atom about
https://brainly.com/question/8806577
#SPJ4
Anything detected with the five senses is considered an
Answers
Answer:
sight, taste, touch, hearing and smell
Explanation:
Anything detected with the five senses is considered a sensory perception or sensory experience
Our five senses are sight (vision), hearing (audition), taste (gustation), touch (tactile perception), and smell (olfaction). These senses allow us to perceive and interpret information from the external environment.
When stated as "detected with the five senses," which means gather information about the world around us through these sensory experiences.
For example:
1. Sight (Vision): We perceive visual information through our eyes, allowing us to see colors, shapes, and movements.
2. Hearing (Audition): We perceive auditory information through our ears, allowing us to hear sounds and distinguish between different tones and pitches.
3. Taste (Gustation): We perceive taste sensations through our taste buds on the tongue, allowing us to distinguish between sweet, sour, salty, bitter, and umami flavors.
4. Touch (Tactile Perception): We perceive tactile sensations through our skin, allowing us to feel textures, pressure, temperature, and pain.
5. Smell (Olfaction): We perceive smells through our olfactory system, located in our nose, allowing us to detect and identify various scents and odors.
All the sensory experiences , whether through sight, hearing, taste, touch, or smell, contribute to our understanding of the world and our surroundings.
Learn more about sensory perceptions here:
https://brainly.com/question/30931333
#SPJ7
Which pair could be genetic clones?
look at the photo

Answers
Answer:
cow female
Explanation: