how many microliters of 1.000 mnaoh solution must be added to 25.00 ml of a 0.1000 m solution of lactic acid ( ch3ch(oh)cooh or hc3h5o3 ) to produce a buffer with ph = 3.75?
Answers
First, we need to calculate equation the concentration of CH3CH(OH)COO- and HCH3CH(OH)COOH needed to produce a buffer solution at a given pH.
We will use the Henderson-Hasselbalch equation for this purpose. Henderson -Hasselbalch are equatio pH = pKa + log [CH3CH(OH)COO-] / [HCH3CH(OH)COOH]pH = 3.75 (given)pKa for lactic acid (HC3H5O3) =
We can assume that the volume of the resulting buffer solution is 25.00 ml (the same as the original volume of lactic acid), so we will add only a tiny amount of NaOH to it. The concentration of NaOH is given as 1.000 M.
To know more about equation Visit;
https://brainly.com/question/27984374
#SPJ11
Related Questions
A compound with the formula C6H12 may or may not be a saturated hydrocarbon.
a. true
b. false
Answers
A compound with the formula C6H12 is not considered a Saturated hydrocarbon.
Why is C6H12 isn't considered a Saturated hydrocarbon?
The ring's presence demonstrates that it is unsaturated. Keep in mind that the general formula for aliphatic hydrocarbons, CnH2n+2, serves as the foundation for its saturation. A chemical is unsaturated if it does not meet this requirement.
Example:
Hexane (C6H14)
C = 6; H = 14 = 2(6) + 2
resulting in hexane becoming saturated.
Cyclohexane(C6H12)
C = 6 and H = 12 do not equal 14 (x)!
cyclohexane is an unsaturated molecule as a result.
Cycloalkanes have the general formula C2H2n as well.
Hence, the given statement is false.
Learn more about the hydrocarbons here,
https://brainly.com/question/17578846
# SPJ4
PLEASE! I HAVE 20 MINS LEFT :( Two aqueous solutions of AgNO3 and NaCl are mixed. Which of the following diagrams best represents the mixture? For simplicity, water molecules are not shown (Ag + = gray, Cl- = orange, Na + = green, NO ^ - 3 = blue) PLEASE I NEED HELP I ONLY HAVE 15 MINS PLS :'((

Answers
Answer:
diagram C best represents the mixture
The half-life of a certain chemical in the human body for a healthy adult is approximately 6 hr. a)What is the exponential decay rate b How long will it take 94% of the chemical consumed to leave the body? aThe decay rate of the chemical is% (Round to one decimal place as needed.) bIt will takehr. (Round to one decimal place as needed.)
Answers
The exponential decay rate for healthy human body is 0.1155 and the time taken by 94% of the chemical consumed by the body to leave is 11.5 hours.
a) The exponential decay rate, often denoted as λ (lambda), can be calculated using the formula:
\(\lambda = \dfrac{ ln(2)} {t^{\frac{1}{2}}}\)
where ln represents the natural logarithm and \(t^\frac{1}{2}\) is the half-life of the chemical.
Substituting the given half-life value:
λ = ln(2) / 6
Using a calculator, we find:
λ ≈ 0.1155
So, the exponential decay rate is approximately 0.1155.
b) To calculate the time it takes for 94% of the chemical to leave the body, we can use the formula for exponential decay:
\(N(t) = N_{o} \times e^{-\lambda t}\)
where N(t) is the amount of chemical remaining at time t, N₀ is the initial amount of chemical, λ is the decay rate, and t is the time elapsed.
We want to find the time at which N(t) is 94% of N₀, which means:
0.94N₀ = N₀ \(\times e^{-\lambda t}\)
Cancelling out N₀:
0.94 = \(e^{-\lambda t}\)
Taking the natural logarithm of both sides:
ln(0.94) = -λt
Substituting the value of λ we found earlier:
ln(0.94) = -0.1155t
Now, solving for t:
t = ln(0.94) / -0.1155
solving the above equation, we get:
t ≈ 11.46
Therefore, the exponential decay rate for healthy human body is 0.1155 and it will take approximately 11.5 hours for 94% of the chemical consumed to leave the body.
Learn more about exponential decay here:
https://brainly.com/question/20674691
#SPJ4
Complete question: The half-life of a certain chemical in the human body for a healthy adult is approximately 6 hr. a)What is the exponential decay rate b How long will it take 94% of the chemical consumed to leave the body?
13. Why are wind and solar power called renewable energy?
HELPP PLZ
Answers
Answer:
It is natural energy coming from nature . Natural energy can be made without the help of humans.
Explanation:
why is copper different than copper ore gives me reasons. Do this asap
Answers
Answer:
copper cathodes are normally referred to when talking about copper. Cathode is the purest form of copper and is the feedstock used to produce copper wire, cable, sheet, strip, tube, etc.
Explanation:
Come up with another way to separate certain mixture?
Answers
Answer:
Oil
Explanation:
Which property would xenon most likely have? it is stable. it is a solid. it is flammable. it is silver in color.
Answers
Answer:
A. It is stable
Explanation:
Ed22
Stability is the property that would xenon most likely to have. Therefore, the correct option is option A.
What is xenon?Chemically speaking, xenon is a gas with the atomic number 54 and the symbol Xe. It is a thick, colourless, and odourless noble gas that is present in minute levels in Earth's atmosphere. It may go through a few chemical reactions, such as the creation of xenon hexafluoroplatinate, which is the initial noble gas combination to be created, despite typically being unreactive.
Flash lamps, arc lamps, and general anaesthetics all employ xenon. The early laser designs utilised xenon flash lamps for pumps and a xenon monomer molecule (Xe2) for the lasing medium in the initial excimer laser design. Stability is the property that would xenon most likely to have.
Therefore, the correct option is option A.
To know more about xenon, here:
https://brainly.com/question/5516586
#SPJ7
How many moles are in 68 grams of copper
Answers
Answer:
1.07 moles
Explanation:
\( mole = \frac{mass}{molar \: mass} \)
\(molar \: mass \: = 63.54\)
\(mole = \frac{68}{63.54} \)
\(mole = 1.07 \: moles\)
A candle wax is mainly a compound consisting of two elements. Name the two elements
Answers
The elements consisting of the candle wax compound are Hydrogen (H) and Carbon (C).
Candle wax is mainly a hydrocarbon. A hydrocarbon is a compound that exclusively contains carbon ad hydrogen. Hydrogen atoms are present in different configurations in the carbon framework in hydrocarbons.
All waxes are necessarily made up of hydrocarbons. They produce carbon dioxide and water vapors on burning. Hydrocarbons are considered the principal constituents of petroleum and natural gas.
The general formula of the candle wax is represented as CₙH₂ₙ₊₂, where n is the number of atoms.
Candle wax is thus mainly a hydrocarbon containing two elements that are- hydrogen and carbon.
To learn more about Hydrocarbons, refer
https://brainly.com/question/17578846
difference between soft water and hard water in table
Answers
Answer:
Here is ur answer
Hope it helps!


Hard water can not be used for laundry while soft water can be used for laundry.
Types of waterThere are basically two types of water, hard water and soft water. The following is a comparison of the properties of hard water and soft water.
Hard water do not form lather with soap while soft water forms lather with soap.Hard water contains calcium and magnesium ions while soft water does not contain calcium and magnesium ions.Hard water forms white patches when boiled in a kettle but soft water does not do that.Hence hard water can not be used for laundry while soft water can be used for laundry .
Learn more about hard water: https://brainly.com/question/6946622
How many mol of Cl are in 10.0 g of Cl? The molar mass of Cl is 35.45 g/mol
Answers
Answer:
\(\boxed {\boxed {\sf 0.282 \ mol \ Cl}}\)
Explanation:
The molar mass, or mass of 1 mole of a substance, is used to convert grams to moles. We are given the molar mass of chlorine. It is 35.45 grams per mole.
We convert using dimensional analysis, so we must set up a conversion factor using the molar mass.
\(\frac { 35.45 \ g \ Cl}{1 \ mol \ Cl}\)
We are converting 10.0 grams of chlorine to moles, so we must multiply the conversion factor by this value.
\(10.0 \ g \ Cl* \frac { 35.45 \ g \ Cl}{1 \ mol \ Cl}\)
Flip the conversion factor. The result is equivalent and allows us to cancel the units of grams of chlorine.
\(10.0 \ g \ Cl* \frac {1 \ mol \ Cl}{ 35.45 \ g \ Cl}\)
\(10.0 * \frac {1 \ mol \ Cl}{ 35.45 }\)
\(\frac {10.0 }{35.45} \ mol \ Cl\)
\(0.282087447 \ mol \ Cl\)
The original value of grams (10.0) has 3 significant figures, so our answer must have the same. For the number we found, that is the thousandth place. The 0 in the ten-thousandth place tells us to leave the 2 in the thousandth place.
\(0.282 \ mol \ Cl\)
Approximately 0.282 moles of chlorine are in 10.0 grams of chlorine.
The experimental apparatus represented above is used to demonstrate the rates at which gases diffuse. When the cotton balls are placed in the ends of a tube at the same time, the gases diffuse from each end and meet somewhere in between, where they react to form a white solid. Which of the following combinations will produce a solid closest to the center of the tube?
a. HCI and CH3NH2
b. HCl and NH3
c. HBr and CH3NH2
d. HBr and NH3
Answers
Which is the only subatomic particle that is directly involved in chemical reactio mns?
Answers
Electrons is the only subatomic particle that is directly involved in chemical reactions.
Electrons is the only subatomic particle that is directly involved in chemical reactions. It is a negatively charged specie which lies in the orbit of atoms and hence gets involved in chemical reaction by gaining and losing of electrons.
Other subatomic particles like Protons and Neutrons lie in the nucleus hence it is not possible to get involved in chemical reaction.
They do not involved in the transfer of species in the reaction.
Electrons are the particles present in the orbits of atom lying close to the nucleus wherein the electron closest to nucleus is most difficult to remove and lying farthest from nucleus is easiest to remove due to the force applied by the nucleus of the negatively charged entity.
Learn more about Nucleus here, https://brainly.com/question/23366064
#SPJ4
electrolysis of aqueous copper(2) sulfate half ionic equation
Answers
Answer:
Copper is less reactive than hydrogen, so copper (Cu) is produced at the negative electrode. The half equation is: Cu2+ + 2e- → Cu
The hydroxide ion is more reactive than the sulphate ion, therefore this forms water (H2O) and oxygen at the positive electrode.
How many atoms or molecules are in 5.0 moles of the following?
a. O
b. N
c.MgCl₂
d. C₂H₃NO
Answers
Answer:
They all have the same number of molecules or atoms which is 3.011*10²⁴ molecules or atoms.
5 moles of O = 3.011*10²⁴ molecules or atoms
5 moles of N = 3.011*10²⁴ molecules or atoms
5 moles of MgCl2 = 3.011*10²⁴ molecules or atoms
5 moles of C2H3NO = 3.011*10²⁴ molecules or atoms.
Explanation:
a)
5 moles of O = ?
1 mole of any substance is equal to Avogadro's number which is equal to 6.022×10²³molecules or atoms
From the question above,
5 moles of O = 5 × 6.022*10²³ = 3.011×10²⁴atoms or molecules.
b)
5 moles of N
From the same principle or fundamentally stated fact above,
1 mole of any substance = 6.022*10²³molecules or atom
5 moles of N = 5 × 6.024
2*10²³ = 3.011*10²⁴atoms or molecules.
c)
5 moles of MgCl₂
Same principle we used in a and b,
1 mole of any substance = 6.022*10²³ atoms or molecules
5 moles of MgCl2 = 5.0×6.022*10²³ = 3.011*10²⁴ atoms or molecules.
d)
5 moles of C₂H₃NO = ?
1 mole of C₂H₃NO = 6.022*10²³molecules or atoms
5 moles = x molecules or atoms
x = 5 × 6.024
2*10²³ = 3.011*10²⁴ atoms or molecules
What this proves is that no matter the compound, molecule or element, as long as they have equal amount of moles, they'll have the same number of atoms, molecules or particles.
Answer:
a. 3.011x10²⁴ atoms of O in 5 moles.
b. 3.011x10²⁴ atoms of N in 5 moles.
c. 3.011x10²⁴ atoms of MgCl₂ in 5 moles.
d. 3.011x10²⁴ atoms of C₂H₃NO in 5 moles
Explanation:
A mole is defined as the number of atoms in exactly 12.000g of ¹²C.
This number is equal to 6.022x10²³. That means 1 mole is equal to 6.022x10²³.
Thus:
a. O . 5 moles of oxygen (An atom) are:
5 moles O ₓ (6.022x10²³ atoms of O / mole) = 3.011x10²⁴ atoms of O in 5 moles
b. N . Also, 5 moles of nitrogen are:
5 moles N ₓ (6.022x10²³ atoms of N / mole) = 3.011x10²⁴ atoms of N in 5 moles
c. MgCl₂ . Magnesium chloride is a molecule. Again, 1 mole of MgCl₂ contains 6.022x10²³ molecules and 5 moles are:
5 moles MgCl₂ ₓ (6.022x10²³ molecules of MgCl₂ / mole) = 3.011x10²⁴ atoms of MgCl₂ in 5 moles
d. C₂H₃NO. 5 moles of C₂H₃NO are:
5 moles C₂H₃NO ₓ (6.022x10²³ molecules of C₂H₃NO / mole) = 3.011x10²⁴ atoms of C₂H₃NO in 5 moles
As you can see, number of molecules of 1 mole doesn't depend on the nature of the substance.
Mg +
Cl₂
->
balance the reaction
Answers
Answer:
Magnesium chloride (MgCl₂)
Explanation:
Given:
Mg + Cl₂
Find:
Balance the reaction
Computation:
Mg + Cl₂ ⇒ MgCl₂
Oxidation-reduction
Magnesium chloride (MgCl₂)
Can someone help me please?
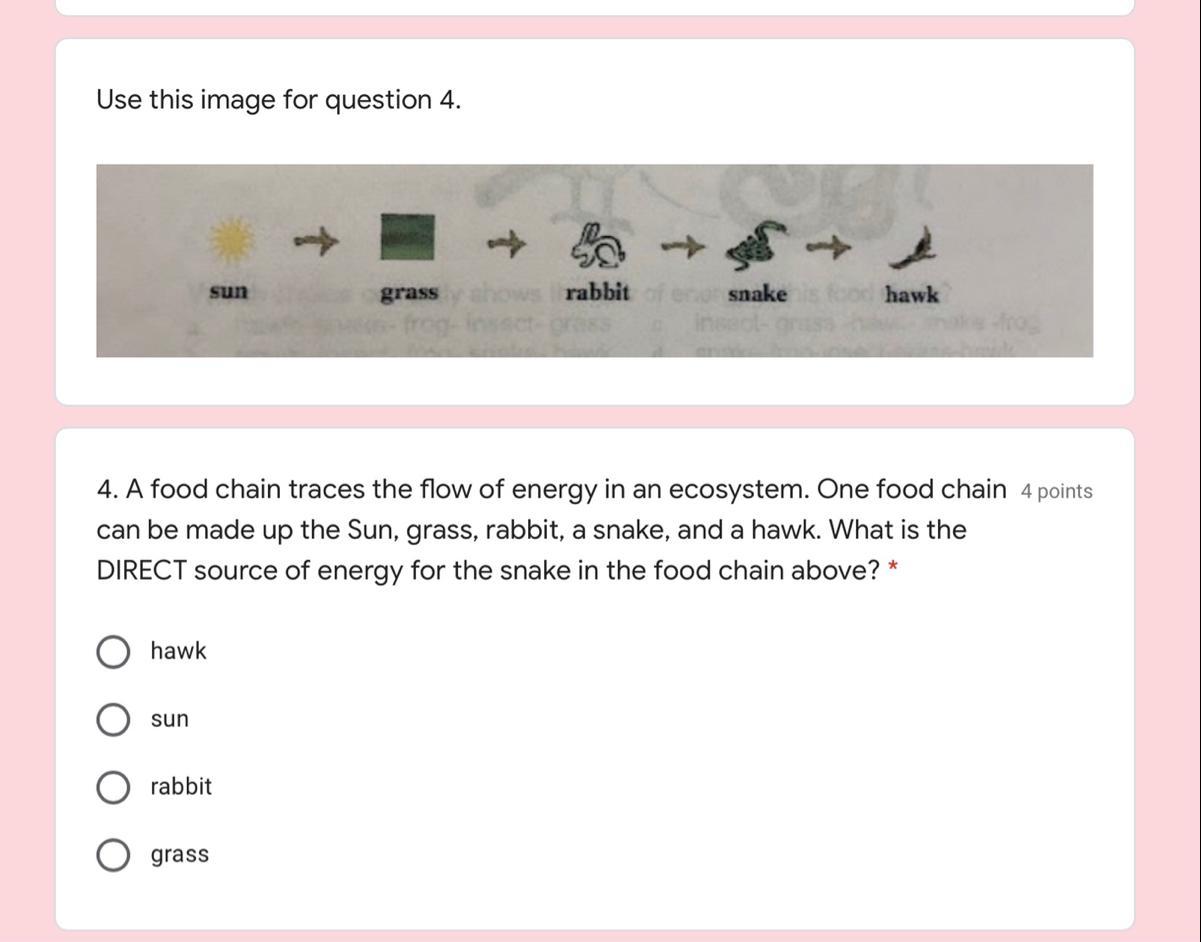
Answers
Answer:
SUN
Explanation:
Hope this helps! Plz mark as brainliest!
And hope it helps
Highly reactive elements that belong to Groups 3-12 that are somewhat reactive and were some of the first discovered
Answers
The statement is incorrect. The elements that belong to Groups 3-12 are known as transition metals. They are characterized by having high melting and boiling points, forming colorful compounds, and displaying a range of oxidation states.
These elements are also known for their high conductivity, strength, and durability, making them useful in various industrial applications. While some transition metals are less reactive than others, they are generally not considered to be highly reactive. Additionally, the discovery of the transition metals occurred over a long period of time, with different elements being identified at different times. Some of the earliest transition metals to be discovered include copper, silver, and gold.
To know more about Highly reactive elements click this link -
brainly.com/question/24863496
#SPJ11
If Liquid diethyl ether (AHvap = 26.5 kJ/mol) is poured into a beaker on a humid day, the ether will evaporate, and frost will form on the beaker. Construct an explanation for the process.
Answers
In comparison to the greater hydrogen bonding in ethanol, diethyl ether molecules are bound together by weak dispersion forces. As a result, one mole of diethyl ether takes less heat to vapourize than one mole of ethanol.
Is diethyl ether a more volatile substance than water?The vapour pressure of diethyl ether at these temperatures is more than 20 times that of water, indicating its volatility.
Diethyl Ether, CH3CH2OCH2CH3, is a highly flammable organic solvent that was also revealed to be one of the earliest anaesthetics. Because it boils at 34.6°C, just below the typical human body temperature, ether evaporates quickly. Since its vapour is denser than air, ether fumes tend to sink into the atmosphere.
learn more about diethyl ether
https://brainly.com/question/14690225
#SPJ1
Calculak pH of the titration of acotic acid by sodiun hydroxide? HA: audic and 0.1M,10 mL NaOH: 0.1M.
Answers
The pH of the titration of acetic acid by sodium hydroxide is 12.
The given question is about calculating the pH of the titration of acetic acid by sodium hydroxide with the given information.
Below is the step-by-step solution to the given problem.
Step 1: Writing down the balanced chemical equation
CH3COOH (aq) + NaOH (aq) → CH3COONa (aq) + H2O
(l)Step 2: Calculating moles of NaOH used
Moles of NaOH = concentration × volume
Moles of NaOH = 0.1 M × 10 mL (convert mL to L)
Moles of NaOH = 0.001 L × 0.1 M = 0.0001 mol
Step 3: Calculating moles of acetic acid
Moles of acetic acid = moles of NaOH (balanced equation)Moles of acetic acid = 0.0001 mol
Step 4: Calculating the initial moles of acetic acid
Initial moles of acetic acid = concentration × volume
Initial moles of acetic acid = 0.1 M × 0.01 L (convert 10 mL to L)
Initial moles of acetic acid = 0.001 mol
Step 5: Calculating the moles of acetic acid that reacted
Moles of acetic acid that reacted = initial moles of acetic acid - moles of acetic acid that remained
Moles of acetic acid that reacted = 0.001 mol - 0.0001 mol = 0.0009 mol
Step 6: Calculating the concentration of acetic acid that remained
Concentration of acetic acid that remained = moles of acetic acid that remained / volume of acetic acid that remained
Concentration of acetic acid that remained = 0.0001 mol / 0.01 L = 0.01 M
Step 7: Calculating the concentration of acetate ions
Concentration of acetate ions = moles of acetate ions / volume of acetate ions
Concentration of acetate ions = 0.0001 mol / 0.01 L = 0.01 M
Step 8: Calculating the concentration of hydroxide ions
Concentration of hydroxide ions = moles of NaOH / volume of NaOH
Concentration of hydroxide ions = 0.0001 mol / 0.01 L = 0.01 M
Step 9: Calculating the pH of the solution
The reaction between acetic acid and sodium hydroxide is a neutralization reaction.
The pH of the solution can be calculated by using the following formula:
pH = 14 - pOH
pOH = -log10 [OH-]pOH = -log10 [0.01]pOH = 2pH = 14 - 2pH = 12
Therefore, the pH of the titration of acetic acid by sodium hydroxide is 12.
To know more about pH, visit:
https://brainly.com/question/2288405
#SPJ11
Can someone help me here please i need this :(
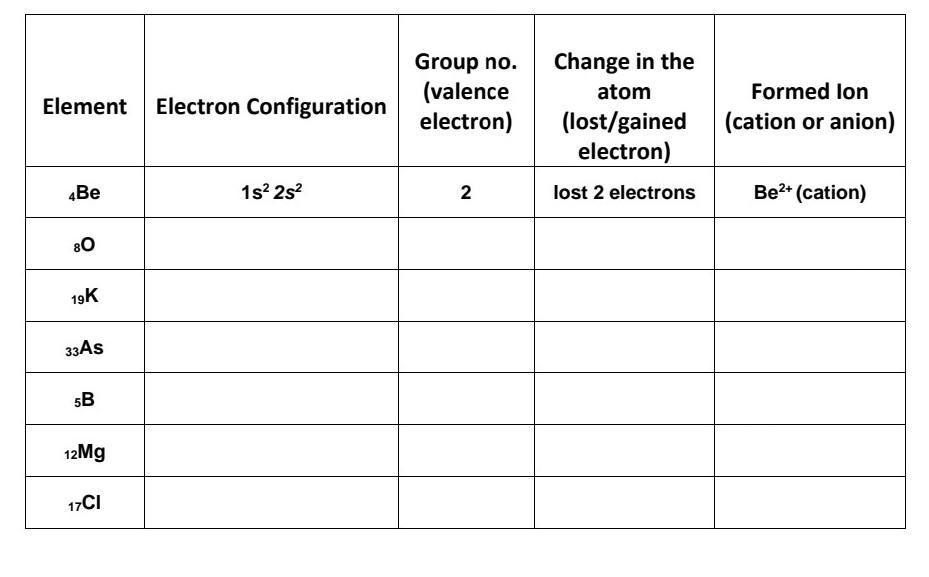
Answers
______________ is the method of energy transfer that involves the flow of fluid materials
Answers
Convection is the method of energy transfer that involves the flow of fluid materials.
Elemental potassium reacts violently with water. The reactionusually results in a lilac-colored flame, fueled by the formation offlammable hydrogen gas, as seen in the reaction below. How manygrams of hydrogen are produced when 43.7 grams of potassiumare tossed into water? Show all work and give your answers tothree significant figures.2K + 2H2O -> 2KOH + H2M(K) = 39.10 g/mol; M(H2O) = 18.01 g/mol; M(KOH) = 56.11 g/mol;M(H2) = 2.02 g/mol
Answers
Explanation:
First, we need to rewrite the equation:
2K + 2H2O -> 2KOH + H2
This equation tells us that 2 moles of K react with 2 moles of H2O and produce 2 moles of KOH and 1 mole of H2.
If we have 43.7 grams of potassium, we can transform it into moles, compare with the ratio between Potassium and Hydrogen (H2) and then, calculate the quantity of hydrogen in grams.
So first, let's transform 43.7 grams of K into moles using the following formula:
moles = mass/molar mass
molar mass of K = 39.10 g/mol
moles = 43.7/39.10
moles = 1.12 moles
So:
2 moles of K --- 1 moles of H2
1.12 moles of K --- x moles of H2
2x = 1.12
x = 1.12/2
x = 0.559 moles of H2
Now let's transform moles into grams using the following formula:
mass = moles x molar mass
molar mass of H2 = 2.02 g/mol
mass = 0.559 x 2.02
mass = 1.13 g
Answer: It is produced 1.13 grams of H2.
How much energy is removed from 10.55 kg of water to lower the temperature from 22.5 C to 3.0 C
Answers
Answer:
Explanation:
To calculate the amount of energy removed from 10.55 kg of water to lower its temperature from 22.5 °C to 3.0 °C, we can use the formula:
Q = m * c * ΔT
where Q is the amount of energy in joules, m is the mass of water in kilograms, c is the specific heat capacity of water (4.184 J/g-K), and ΔT is the change in temperature in degrees Celsius.
First, we need to convert the mass of water from kilograms to grams:
m = 10.55 kg * 1000 g/kg = 10550 g
Next, we need to calculate the change in temperature:
ΔT = 3.0 °C - 22.5 °C = -19.5 °C
Note that we have a negative temperature change because the water is losing heat.
Now, we can plug in the values into the formula:
Q = m * c * ΔT = 10550 g * 4.184 J/g-K * (-19.5 °C) = -820,026 J
The negative sign indicates that energy is being removed from the water, which is consistent with the fact that the temperature is decreasing. Therefore, approximately 820,026 joules of energy are removed from 10.55 kg of water to lower the temperature from 22.5 °C to 3.0 °C.
Using examples, explain which electrochemistry technology you think is the most cost efficient.
Answers
Among various electrochemistry technologies, lithium-ion batteries are considered the most cost-efficient due to their widespread use, decreasing prices, and high energy density.
Lithium-ion batteries have emerged as the dominant technology for energy storage in portable electronics, electric vehicles, and renewable energy systems. They offer a combination of high energy density, long cycle life, and relatively low self-discharge rates compared to other electrochemical technologies. These factors make them highly cost-efficient in a variety of applications.
One example of the cost efficiency of lithium-ion batteries can be seen in the electric vehicle (EV) market. Over the years, advancements in lithium-ion battery technology and increased production scale have led to significant cost reductions. This has resulted in a decline in the prices of EVs, making them more accessible to consumers. The cost efficiency of lithium-ion batteries has also been demonstrated in the renewable energy sector. Energy storage systems based on lithium-ion batteries allow for efficient integration of intermittent renewable energy sources, such as solar and wind power, into the grid. This helps stabilize the grid and reduce reliance on fossil fuels.
Furthermore, the high energy density of lithium-ion batteries enables compact and lightweight designs, making them suitable for portable electronics like smartphones and laptops. This not only enhances user convenience but also contributes to cost efficiency by reducing material and transportation costs. Additionally, the long cycle life of lithium-ion batteries ensures durability and longevity, further enhancing their cost efficiency as they require fewer replacements over their lifespan.
Learn more about lithium-ion batteries here:
https://brainly.com/question/13651147
#SPJ11
4) When solid sodium nitrate is heated, solid sodium nitrite and oxygen gas
(02) are produced. Write the balanced chemical equation for this chemical
reaction using the correct chemical formulas and include conditions (s, l, g, or
aq). Put your answer in the box below.
Write the balanced chemical equation and state what type of chemical reaction this is.
Answers
Answer: The balanced chemical equation is \(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\) and it is a decomposition chemical reaction.
Explanation:
A chemical reaction in which single compound is decomposed into two or more species is called a decomposition reaction.
For example, \(NaNO_{3}(s) + Heat \rightarrow NaNO_{2}(s) + O_{2}(g)\)
As the compound \(NaNO_{3}\) is decomposing to given two different substances. So, it is a decomposition reaction.
Here, the number of atoms on reactant side are as follows.
Na = 1N = 1O = 3The number of atoms on product side are as follows.
Na = 1N = 1O = 4To balance this equation, multiply \(NaNO_{3}\) by 2 on reactant side and multiply \(NaNO_{2}\) by 2 on product side. Therefore, the equation will be rewritten as follows.
\(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\)
Here, number of atoms on reactant side are as follows.
Na = 2N = 2O = 6The number of atoms on product side are as follows.
Na = 2N = 2O = 6Hence, this equation is balanced.
Thus, we can conclude that the balanced chemical equation is \(2NaNO_{3}(s) + Heat \rightarrow 2NaNO_{2}(s) + O_{2}(g)\) and it is a decomposition chemical reaction.
Which of the following descriptions apply to valence electrons?
Select all that apply.
They are electrons in the highest occupied principal energy level of an atom.
They participate in chemical bonds.
They do not determine how reactive an atom will be.
They must always be paired with another electron.
Answers
They were electrons in an atom's greatest primary energy level, which is occupied. In chemical bonds, they take part.
How do chemical bonds work?Atoms in molecules are held together by chemical bonds. Electrostatic forces between negatively charged electrons and positively charged atomic nuclei, whose locations in space are governed by quantum mechanics, give rise to bonds.
What makes chemical bonds so crucial?One of the most fundamental principles of chemistry, chemical bonding helps to explain other ideas like molecules and reactions. Without it, scientists would be unable to explain why atoms are drawn to one another or how products are created during a chemical process.
To know more about chemical bond visit:
https://brainly.com/question/15444131
#SPJ13
What approximate volume of the oxytocin solution with the 10 mM Zn2+ additive was analyzed if 2.2 à 10â6 moles of oxytocin acetate (MW = 1067 g/mol) were recovered from the sample after 4 weeks at 50 °C?
Answers
moles = mass / molar mass We know the moles of oxytocin acetate recovered (2.2 x 10^-6 moles) and its molar mass (1067 g/mol). We need to find the mass of the oxytocin acetate in the solution, and from there we can determine the volume of the solution.
mass = moles x molar mass
mass = 2.2 x 10^-6 moles x 1067 g/mol
mass = 2.3454 x 10^-3 g
Now, we need to take into account the 10 mM Zn2+ additive. We don't know the exact concentration of the oxytocin solution, but we can assume that the 10 mM Zn2+ additive does not significantly change the volume of the solution. Therefore, we can calculate the volume of the solution using the mass of oxytocin acetate and its concentration in the original sample.Let's assume that the original sample had a concentration of 1 mM (this is just an example, the actual concentration could be different). This means that there was 1 mmol of oxytocin acetate per liter of solution. To find the volume of the solution that was analyzed, we can use the following formula:
volume = mass / (concentration x molar mass)
volume = 2.3454 x 10^-3 g / (1 x 10^-3 mol/L x 1067 g/mol)
volume = 2.3454 x 10^-6 L or 2.3454 µL
Therefore, the approximate volume of the oxytocin solution with the 10 mM Zn2+ additive that was analyzed is 2.3454 µL.
To know more about volume please vist :-
https://brainly.com/question/15861918
#SPJ11
The approximate volume of the oxytocin solution with the 10 mM Zn²⁺ additive that was analyzed is 2.3454 µL.
moles = mass / molar mass
We know the moles of oxytocin acetate recovered (2.2 x 10⁻⁶ moles) and its molar mass (1067 g/mol).
mass = moles x molar mass
mass = 2.2 x 10⁻⁶ moles x 1067 g/mol
mass = 2.3454 x 10⁻³ g
Utilizing the mass of oxytocin acetate and its concentration in the first sample, we can determine the volume of the solution. Assume for the sake of argument that the first sample had a concentration of 1 mM (the real concentration may have been different).
This indicates that each litre of solution contained 1 mmol of oxytocin acetate. Using the following formula, we can get the volume of the solution that was examined:
volume = mass / (concentration x molar mass)
volume = 2.3454 x 10⁻³ g / (1 x 10⁻³ mol/L x 1067 g/mol)
volume = 2.3454 x 10⁻⁶ L or 2.3454 µL
Therefore, the approximate volume of the oxytocin solution with the 10 mM Zn²⁺ additive that was analyzed is 2.3454 µL.
Learn more about Volume of oxytocin:
https://brainly.com/question/28854491
#SPJ4
BRAINLIEST !!! HELP PLZ

Answers
Answer:
Hcl Answer
Explanation:
may be this helpful!
What is a picture of homologous pairs of chromosomes and sex chromosomes?
nuclear DNA
stereotype
karyotype
allele
Answers
Hope this helps :)