How many molecules are present in 3HNO4?
1) 14
2) 18
3) 3
4) 4
Answers
Answer:
2
Explanation:
If you take H and N, that is two molecules right off the bat. If you take O4, that makes 6. Take the total of all 6, and multiply it by 3, with equals 18.
3HNO4 = 18
Related Questions
calcium sulfite decomposes when heated to form calcium oxide and sulfur dioxide. how many grams of sulfur dioxide is produced from 3 moles of calcium sulfite?
Answers
calcium sulfite decomposes when heated to form calcium oxide and sulfur dioxide. This involves a Decomposition Reaction. 192.19 gram of sulfur dioxide is produced from 3 moles of calcium sulfite.
Sulfite is the name given to the SO32− anion. So the reactant is composed of calcium cations, Ca 2+ and sulfite anions, SO 32−. Since calcium cations have a 2+ charge, and oxygen anions have a 2− charge, & calcium oxide will simply be
Ca2 + O2− ⇒ Ca2O2 ⇒ Ca O
Sulphur dioxide is a covalent compound. it contains one sulphur atom. Sulphur has no prefix in the name of the compound two oxygen atoms oxygen has a di- prefix in the name of the compound. Therefore, you can say that sulfur dioxide is SO2.
This gives Ca SO3 (s] → Ca O (s] +SO 2(g]↑.
sulfur dioxide is produced from 3 moles of calcium sulfite.
so, 3 mole of CaSO3 * 1 mole of SO2 / 1 mole of Ca SO3
= 3 mole of SO2 = 192.19 grams of SO2
To learn more about Decomposition Reaction please visit:
https://brainly.com/question/21491586
#SPJ4
The health care provider orders KCL 30 mEq. The medication is available in a unit dose package labeled: KCL 60 mEq/10 mL. The medicine cup is marked teaspoons. How many teaspoons will the nurse administer? tsp
Answers
the nurse will administer approximately 1 teaspoon (tsp) of the medication.1 teaspoon (tsp) is approximately equal to 5 mL.
How many teaspoons will the nurse administer?To determine the number of teaspoons the nurse should administer, we need to calculate the equivalent volume of 30 mEq of KCL using the provided concentration of 60 mEq/10 mL.
First, we'll find the ratio of milliequivalents (mEq) to milliliters (mL) in the given concentration:
60 mEq / 10 mL = 6 mEq/mL
Next, we can set up a proportion to find the volume (in mL) that corresponds to 30 mEq:
6 mEq/mL = 30 mEq / X mL
To solve for X, we can cross-multiply:
6X = 30 * 1
6X = 30
X = 30 / 6
X = 5 mL
Since the medication cup is marked in teaspoons, we need to convert 5 mL to teaspoons.
1 teaspoon (tsp) is approximately equal to 5 mL.
Therefore, the nurse will administer approximately 1 teaspoon (tsp) of the medication.
Learn more about measurement
brainly.com/question/28913275
#SPJ11
What is the absolute smallest thing we can break all matter down into without loosing it's property?
Answers
Answer:
Protons and neutrons can be further broken down: they're both made up of things called “quarks.” As far as we can tell, quarks can't be broken down into smaller components, making them the smallest things we know of.
Explanation:
Determine if the following reaction is a redox reaction. Use evidence from the equation to explain your reasoning.

Answers
A redox reaction is a chemical reaction in which one or more of the reacting species undergoes oxidation and one or more undergoes reduction. An oxidizing agent is an element or compound that oxidizes another substance, while a reducing agent is an element or compound that reduces another substance.
The following reaction is a redox reaction based on the following evidence: 2Al + 3FeO → Al2O3 + 3Fe2+ In this reaction, Fe is being reduced because the FeO is changing to Fe2+. Additionally, the Al is being oxidized because it is losing electrons and forming Al2O3. Therefore, the reaction is a redox reaction. Let us take a look at the oxidation state of the elements in the given equation. Oxidation state of Al: (2) for the reactant and (3+) for the product. Oxidation state of Fe: (2+) for the reactant and (2+) for the product. Oxidation state of O: (-2) for the reactant and (-2) for the product. We can tell that oxidation is happening because of the increase in the oxidation state of Al from 2 to 3+. We can tell that reduction is happening because of the decrease in the oxidation state of Fe from 2+ to 2. As a result, the given equation is a redox reaction.For such more question on oxidizes
https://brainly.com/question/14041413
#SPJ8
A person trapped outside during a thunderstorm should
lie in a ditch or other low-lying area.
get out of a car and take shelter under a tree.
stay away from trees, water, and tall objects.
run away from the thunderstorm as quickly as possible.
Answers
Answer:
C stay away from trees, water, and tall objects.
Explanation:
took the quiz and got it right :)
A person trapped outside during a thunderstorm should stay away from the following:
TreesTall objectsWaterWhat is Thunderstorm?This is defined as a rain shower during which you hear thunder with a corresponding lightning.
It is best to avoid trees, water, and tall objects during thunderstorms to prevent accidents and death.
Read more about Thunderstorms here https://brainly.com/question/1779066
whats the molar mass of maganese hydroxide
Answers
The molar mass of a compound is the sum of the atomic masses of all the atoms present in one molecule of the compound. the molar mass of manganese hydroxide is 88.94 g/mol.
To calculate the molar mass of manganese hydroxide, we need to find the atomic masses of each of the elements and add them up in the correct proportions.
The atomic mass of manganese (Mn) is 54.94 g/mol.
The atomic mass of hydrogen (H) is 1.01 g/mol, and the atomic mass of oxygen (O) is 16.00 g/mol.
The formula for manganese hydroxide is Mn(OH)₂, which means there are two hydroxide ions (OH⁻) present for every one manganese atom.
To calculate the molar mass of manganese hydroxide, we add up the atomic masses of all the atoms present in one molecule of the compound: Molar mass of Mn(OH)₂ = (atomic mass of Mn) + 2(atomic mass of O) + 2(atomic mass of H) = 54.94 g/mol + 2(16.00 g/mol) + 2(1.01 g/mol) = 88.94 g/mol
Therefore, the molar mass of manganese hydroxide is 88.94 g/mol.
For more questions on manganese hydroxide
https://brainly.com/question/15516767
#SPJ8
How does a conjugate acid differ from its conjugate base?
Answers
The conjugate acid is differ from its conjugate base as, conjugate acid is formed by strong base whereas conjugate base is formed by strong acid.
conjugate base is differ from conjugate acids by the presence of the proton. The conjugate acid is formed when proton is added to the bases whereas conjugate bases is formed when proton is released by the acids.
Example of corrugate acids are given below.
\(NH_{3}\) → \(NH_{2} ^{-} +H^{+}\)
In the above example \(NH_{2} ^{-}\) is conjugate acids.
Learn about conjugate acid
https://brainly.com/question/12883745
#SPJ4
the mole fraction of carbon dioxide in dry air near sea level is 0.000375, where the molar mass of carbon dioxide is 44.010. the partial pressure of carbon dioxide when the total atmospheric pressure (dry air) is 97.5 kpa is
Answers
The partial pressure of carbon dioxide when the total atmospheric pressure (dry air) is 97.5 kPa is approximately 15.489375 Pa.
To find the partial pressure of carbon dioxide, we can use the mole fraction and the total atmospheric pressure.
Given that the mole fraction of carbon dioxide in dry air near sea level is 0.000375, we can calculate the partial pressure of carbon dioxide as follows:
1. Calculate the moles of carbon dioxide:
Moles of CO2 = Mole fraction * Total moles of gas
Since the mole fraction is 0.000375 and the total moles of gas is not provided, we can assume it to be 1 mole for simplicity:
Moles of CO2 = 0.000375 * 1 = 0.000375 moles
2. Calculate the partial pressure of carbon dioxide:
Partial pressure of CO2 = Moles of CO2 * Molar mass of CO2 * Total atmospheric pressure
The molar mass of carbon dioxide is given as 44.010 g/mol, and the total atmospheric pressure is 97.5 kPa (kiloPascals).
However, we need to convert the pressure to the appropriate units for the molar mass:
1 kPa = 1000 Pa
1 Pa = 1 N/m^2
1 atm = 101325 Pa
1 atm = 101.325 kPa
So, the total atmospheric pressure in Pascals is 97.5 * 1000 = 97500 Pa.
Now, we can calculate the partial pressure of carbon dioxide:
Partial pressure of CO2 = 0.000375 moles * 44.010 g/mol * 97500 Pa
Partial pressure of CO2 = 15.489375 Pa
Learn more about partial pressure here :-
https://brainly.com/question/30114830
#SPJ11
draw the major product formed when the given epoxide reacts with aqueous acid. an epoxide where carbon 1 has an ethyl and hydrogen substituent and carbon 2 has two methyl groups. this reacts with h 3 o plus and water.
Answers
An epoxide is a heterocyclic organic compound with an oxygen ring. The acid protonates the oxygen, which opens the ring and starts the process.
What exactly is carbocation?
A carbocation is a molecule with three bonds and a positively charged carbon atom. To put it simply, they are essentially carbon cations. It was previously known as carbonium ion. A carbocation is any even-electron cation having a significant positive charge on the carbon atom.
Because of an incomplete octet, carbon cations are particularly reactive and unstable in general.
To put it simply, carbocations do not meet the octet rule because they lack eight electrons.
According to the question, carbon hybridization in carbocation will be sp2 with a trigonal planar structure. There is also an empty p orbital, suggesting that it is electron-deficient. Carbon contains six electrons in its valence shell. As a result, it is an electron-deficient species, also known as an electrophile.
An epoxide is a heterocyclic organic compound with an oxygen ring. The acid protonates the oxygen, which opens the ring and starts the process. A carbocation is now generated, which is quickly attacked by a water molecule.
To learn more about hybridization follow the given link: https://brainly.com/question/353992
#SPJ4
An epoxide is a heterocyclic organic compound with an oxygen ring. The acid protonates the oxygen, which opens the ring and starts the process.
What exactly is carbocation?
A carbocation is a molecule with three bonds and a positively charged carbon atom. To put it simply, they are essentially carbon cations. It was previously known as carbonium ion. A carbocation is any even-electron cation having a significant positive charge on the carbon atom.
An epoxide is a heterocyclic organic compound with an oxygen ring? The acid protonates the oxygen, which opens the ring and starts the process. A carbocation is now generated, which is quickly attacked by a water molecule.
To know more about hybridization visit;
https://brainly.com/question/14140731
#SPJ4
he following table contains retention times and width at half height for the peaks of the separation of two compounds, A and B, and an unretained species on a 100.00 cm column. Compound Retention Time Width at Half (D) Height (W1/2) Unretained Species 4.21 min 0.140 min А 5.26 min 0.165 min B 8.22 min 0.210 min Calculate the height of a theoretical plate (HETP) of species A in cm/plate. Hint: You will want to calculate the number of plates first.
Answers
the height of a theoretical plate (HETP) of species A is 1.12 cm/plate.
To calculate the height of a theoretical plate (HETP) of species A, we first need to calculate the number of plates. The formula for the number of plates is:
N = (tR/W1/2)^2
Where N is the number of plates, tR is the retention time, and W1/2 is the width at half height.
Using the values given in the table, we can calculate the number of plates for each compound:
Unretained Species: N = (4.21/0.140)^2 = 110.26 plates
Compound A: N = (5.26/0.165)^2 = 89.56 plates
Compound B: N = (8.22/0.210)^2 = 137.72 plates
Next, we can calculate the HETP using the formula:
HETP = L/N
Where L is the length of the column (100.00 cm).
Using the number of plates calculated above, we can find the HETP for species A:
HETP = 100.00/89.56 = 1.12 cm/plate
Therefore, the height of a theoretical plate (HETP) of species A is 1.12 cm/plate.
learn more about Species here
https://brainly.com/question/29330415
#SPJ11
The decomposition
of dimethyl ether, (CH3)20, at 510°C is a first-order process with a
rate constant of 6.8 x 10+ s¹:
(a) If the initial pressure of (CH3)20, is 135 torr, what is its pressure after 1420 s?
(CH3)2O, (g) → CH, (g) + Hz (g)+CO(g)
(b) Calculate the half-life ?
Answers
If the initial pressure of (CH3)20, is 135 torr, the final pressure after 1420s is 51.4torr .the half life is 1019s.
We know ,
For the 1st order reaction we have the reaction in terms of pressure is
Pₓ = P₀ e⁻kt
lnPₓ = lnP₀ - kt
kt = lnP₀ -lnPₓ = 2.303log P₀/Pₓ
t = (2.303/k) logP₀/Pₓ
Where ,
k = rate constant = 6.8×10⁻4 s⁻1
P₀ = initial pressure = 135torr
Pₓ= final pressure
t = time taken = 1420s
putting the values in the places of the symbol ,
1420 =( 2.303 / 6.8 ×10⁻4 ) log 135/Pₓ
1420 = (2.303×10^4/6.8 ) ×log 135/Pₓ
(1420×6.8 / 2.303×10^4) = log 135/Pₓ
0.41928 = log 135/Pₓ
135/Pₓ = 10^0.41928 = 2.6259
Pₓ = 135/2.6259 = 51.4 torr
Hence , the final pressure after 1420s is 51.4torr .
b) the half life formula is given by ,
T1/2 = 0.693/k = 0.693 / 6.8×10⁻4 = 0.1019×10^4 =1019s
Hence , the half life is 1019s .
Learn more about half life here :
brainly.com/question/1160651
#SPJ13
what must be worn in the laboratory at all times and why
Answers
Wearing appropriate clothing and protective gear in a laboratory is crucial for maintaining a safe and healthy working environment. It is important to follow the guidelines and regulations set by the laboratory to ensure the safety of everyone involved in the research and experiments. By taking these precautions seriously, lab workers can minimize the risk of accidents and injuries and focus on conducting successful experiments and research.
Laboratories are places where scientific experiments and research are conducted, and they require careful handling of hazardous materials, chemicals, and equipment. It is essential to follow certain guidelines to ensure the safety of the individuals working in the lab. One of the most important guidelines is to wear appropriate clothing and protective gear.
The clothing worn in the laboratory must be comfortable, durable, and provide adequate coverage of the body. Loose-fitting clothes, jewelry, and open-toed shoes should be avoided as they can increase the risk of accidents and injuries. Lab coats or aprons should be worn to protect clothing from chemicals, spills, and splashes.
Protective gear such as gloves, safety goggles, and face shields are also necessary in a laboratory. Gloves protect hands from chemical exposure, cuts, and punctures. Safety goggles and face shields protect the eyes and face from chemical splashes, flying debris, and harmful radiation.
Additionally, it is important to tie back long hair and avoid using hair products that could ignite or react with chemicals. Lab workers should also avoid wearing contact lenses as they can trap chemicals and cause eye damage.
Wearing appropriate clothing and protective gear in a laboratory is crucial because it minimizes the risk of accidents and injuries. Chemical spills, splashes, and explosions can cause serious harm to the body, and wearing protective gear can significantly reduce the severity of these injuries. Proper attire and gear can also prevent contamination of samples, equipment, and the environment.
Here you can learn more about laboratory
https://brainly.com/question/27971838#
#SPJ11
which chemical waste situation should always be supervised or performed by an instructor? note that you still may need to alert your instructor about the described incident, even if you clean it up yourself. select one: cleaning up solutions from a titration experiment cleaning up solid residue from a precipitation experiment cleaning up a broken beaker containing sodium chloride solution cleaning up a broken mercury thermometer
Answers
The chemical waste situation that should always be supervised or performed by an instructor is cleaning up a broken mercury thermometer. Mercury is a toxic substance that poses severe health risks, and its vapors can be inhaled or absorbed through the skin.
It is crucial to handle a mercury spill with extreme care and adhere to proper disposal procedures to minimize exposure and prevent environmental contamination.
Although cleaning up solutions from a titration experiment, solid residue from a precipitation experiment, and a broken beaker containing sodium chloride solution are essential tasks, they usually involve lower risks compared to handling mercury. In these cases, students may clean up the waste themselves while following the appropriate safety guidelines, but it is still recommended to alert the instructor about the incident for proper guidance and supervision.The chemical waste situation that should always be supervised or performed by an instructor is cleaning up a broken mercury thermometer. Mercury is a toxic substance that poses severe health risks, and its vapors can be inhaled or absorbed through the skin.
In summary, always prioritize safety and seek your instructor's assistance when dealing with hazardous substances like mercury to ensure proper handling and disposal.
learn more about chemical here
https://brainly.com/question/31052999
#SPJ11
a 20.0-ml sample of 0.150 m ethylamine is titrated with 0.0981 m hcl. what is the ph after the addition of 5.0 ml of hcl? for ethylamine, pkb
Answers
After adding 5.0 mL of 0.0981 M HCl to a 20.0 mL sample of 0.150 M ethylamine, the pH of the solution is approximately 12.003.
To determine the pH after adding 5.0 mL of 0.0981 M HCl to a 20.0 mL sample of 0.150 M ethylamine, we need to consider the dissociation of ethylamine and the resulting acid-base reaction.
Ethylamine (CH3CH2NH2) acts as a weak base and undergoes partial ionization in water. Its conjugate acid is ethylammonium ion (CH3CH2NH3+). The pKb of ethylamine is given as 3.25.
Before the addition of HCl, the ethylamine solution acts as a weak base, and we can calculate the concentration of ethylamine using the initial volume and molarity:
initial moles of ethylamine = (20.0 mL) * (0.150 mol/L) = 3.00 mmol
Since the reaction between ethylamine and HCl is 1:1, the moles of HCl added is:
moles of HCl = (5.0 mL) * (0.0981 mol/L) = 0.4905 mmol
After the reaction, the remaining moles of ethylamine will be:
moles of ethylamine = initial moles of ethylamine - moles of HCl = 3.00 mmol - 0.4905 mmol = 2.5095 mmol
To calculate the concentration of ethylamine after the addition of HCl, we need to consider the new volume:
final volume = initial volume + volume of HCl = 20.0 mL + 5.0 mL = 25.0 mL
Converting to liters:
final concentration of ethylamine = moles of ethylamine / final volume = (2.5095 mmol) / (25.0 mL / 1000) = 100.38 mmol/L
Now, we can determine the pOH of the ethylamine solution using the pKb value:
pOH = pKb - log10(concentration of ethylamine) = 3.25 - log10(100.38) ≈ 1.997
Finally, we can calculate the pH using the relationship:
pH = 14 - pOH = 14 - 1.997 ≈ 12.003
Therefore, the pH after adding 5.0 mL of HCl to the ethylamine solution is approximately 12.003.To determine the pH after adding 5.0 mL of 0.0981 M HCl to a 20.0 mL sample of 0.150 M ethylamine, we need to consider the dissociation of ethylamine and the resulting acid-base reaction.
Ethylamine (CH3CH2NH2) acts as a weak base and undergoes partial ionization in water. Its conjugate acid is ethylammonium ion (CH3CH2NH3+). The pKb of ethylamine is given as 3.25.
Before the addition of HCl, the ethylamine solution acts as a weak base, and we can calculate the concentration of ethylamine using the initial volume and molarity:
initial moles of ethylamine = (20.0 mL) * (0.150 mol/L) = 3.00 mmol
Since the reaction between ethylamine and HCl is 1:1, the moles of HCl added is:
moles of HCl = (5.0 mL) * (0.0981 mol/L) = 0.4905 mmol
After the reaction, the remaining moles of ethylamine will be:
moles of ethylamine = initial moles of ethylamine - moles of HCl = 3.00 mmol - 0.4905 mmol = 2.5095 mmol
To calculate the concentration of ethylamine after the addition of HCl, we need to consider the new volume:
final volume = initial volume + volume of HCl = 20.0 mL + 5.0 mL = 25.0 mL
Converting to liters:
final concentration of ethylamine = moles of ethylamine / final volume = (2.5095 mmol) / (25.0 mL / 1000) = 100.38 mmol/L
Now, we can determine the pOH of the ethylamine solution using the pKb value:
pOH = pKb - log10(concentration of ethylamine) = 3.25 - log10(100.38) ≈ 1.997
Finally, we can calculate the pH using the relationship:
pH = 14 - pOH = 14 - 1.997 ≈ 12.003
The pH after adding 5.0 mL of HCl to the ethylamine solution is approximately 12.003.
To know more about ethylamine refer here
brainly.com/question/8982047#
#SPJ11
The pH after the addition of 5.0 mL of HCl is approximately 5.638.
To calculate the pH after the addition of HCl, we can use the Henderson-Hasselbalch equation, which relates the pH of a solution to the pKa or pKb of the acid or base involved. In this case, ethylamine acts as a base, so we will use the pKb value.
The Henderson-Hasselbalch equation is given by:
pH = pKb + log ([base]/[acid])
Given that the initial volume of ethylamine is 20.0 mL and its concentration is 0.150 M, we can calculate the initial number of moles of ethylamine:
moles of ethylamine = concentration × volume
= 0.150 M × 0.0200 L
= 0.00300 moles
Since ethylamine is a weak base, it reacts with HCl according to the balanced chemical equation:
ethylamine (C₂H₅NH₂) + HCl → C₂H₅NH₃⁺Cl⁻
Using the stoichiometry of the reaction, we find that 1 mole of ethylamine reacts with 1 mole of HCl.
The volume of HCl added is 5.0 mL, which is equivalent to 0.0050 L. The concentration of HCl is 0.0981 M, so we can calculate the moles of HCl added:
moles of HCl = concentration × volume
= 0.0981 M × 0.0050 L
= 0.0004905 moles
Since the reaction between ethylamine and HCl is a 1:1 ratio, the moles of ethylamine that react with HCl are also 0.0004905 moles.
Now, we can calculate the moles of ethylamine remaining:
moles of ethylamine remaining = initial moles of ethylamine - moles of ethylamine reacted with HCl
= 0.00300 moles - 0.0004905 moles
= 0.00251 moles
Next, we can calculate the concentration of ethylamine after the reaction:
concentration of ethylamine = moles of ethylamine remaining / volume of ethylamine
= 0.00251 moles / 0.0200 L
= 0.1255 M
Finally, we can substitute the values into the Henderson-Hasselbalch equation to find the pH:
pH = pKb + log ([base]/[acid])
= pKb + log (0.1255 M / 0.0004905 M)
= pKb + log (255.88)
To determine the pKb value for ethylamine, we can use the relationship pKa + pKb = 14 for a conjugate acid-base pair. Since the pKa of ethylammonium ion (C₂H₅NH₃⁺) is given as 10.77, we can find the pKb:
pKb = 14 - pKa
= 14 - 10.77
= 3.23
Substituting this value into the Henderson-Hasselbalch equation, we find:
pH = 3.23 + log (255.88)
Using logarithmic
properties, we can evaluate the expression inside the logarithm:
pH ≈ 3.23 + 2.408
≈ 5.638
Therefore, the pH after the addition of 5.0 mL of HCl is approximately 5.638.
To know more about Henderson-Hasselbalch equation refer here:
https://brainly.com/question/31495136#
#SPJ11
1. Which of the following species determines the chemical properties of an atom?
A. Electron
B. Neutron
C. Nucleus
D. Proton
2. The following glasswares are used to measure the volume of liquids except
A. graduated beaker
B. pipette
C. test tube
D. burette
3. Pauli exclusion principle is related to
A. quantity of electrons in the valence shell
B. filling the orbitals with lower energy first
C. the filling of degenerated orbitals
D. quantum numbers of electrons
Answers
Explanation:
The number of electrons, in turn, determines the chemical properties of the atom. Protons contribute to the mass of an atom and provide the positive charge to the nucleus. The number of protons also determines the identity of the element
Answer:
Question 1: (A)
The number of electrons determine the chemical properties of an atom.
Question 2: (C)
Test tube cannot measure the volume of liquids rather they are used in chemical reactions
Question 3: (D)
Pauli's Exclusion Principle states that no two electrons in the same atom can have identical values for all four of their quantum numbers.
what is the concentration of hcl when you dilute 17.5 ml of a 3.31 m hcl stock solution to 159 ml? round your answer to 3 decimal places. do not include units.
Answers
The concentration of the diluted HCl solution is 0.363 M, rounded to 3 decimal places.
When a stock solution is diluted, the number of moles of the solute (in this case, HCl) remains constant. We can use the following equation to find the concentration of the diluted solution:
M1V1 = M2V2
where M1 is the initial concentration of the stock solution, V1 is the volume of the stock solution used, M2 is the final concentration of the diluted solution, and V2 is the final volume of the diluted solution.Substituting the given values, we get:
(3.31 M) × (17.5 mL) = M2 × (159 mL)
Solving for M2, we get:
M2 = (3.31 M × 17.5 mL) / 159 mL = 0.363 M.
For such more questions on Diluted HCl:
https://brainly.com/question/24613812
#SPJ11
CsH16 +12028CO2 +8H₂O
What is the ratio of octene (C8H16) to
oxygen in the reaction?
Answers
The ratio of octene to oxygen is 1:12.
To determine the ratio of octene (C8H16) to oxygen (O2) in the given reaction, we need to examine the balanced chemical equation. However, the equation you provided does not seem to be balanced. The coefficients for each compound must be determined to achieve a balanced equation before we can calculate the desired ratio.
Assuming you meant the combustion reaction of octene, a balanced equation would be:
C8H16 + 12O2 → 8CO2 + 8H2O
From the balanced equation, we can see that for every 1 mole of octene (C8H16), we require 12 moles of oxygen (O2) to completely react.
This means that for every 1 mole of octene, we need 12 moles of oxygen to fully combust the octene and produce the corresponding amounts of carbon dioxide (CO2) and water (H2O) as shown in the balanced equation.
For such more questions on combustion
https://brainly.com/question/13251946
#SPJ8
A World Health Organization study of health in various countries reported that in Canada, systolic blood pressure readings have a mean of 121 and a standard deviation of 16 . A reading above 140 is considered to be high blood pressure. Complete parts a through d below. a. What is the z− score for a blood pressure reading of 140 ? z= (Round to two decimal places as needed.) b. If systolic blood pressure in Canada has a normal distribution, what proportion of Canadians suffers from high blood pressure? The proportion of Canadians with high blood pressure is (Round to four decimal places as needed.) c. What proportion of Canadians has systolic blood pressure in the range from 100 to 140 ? The proportion with systolic blood pressure between 100 and 140 is (Round to four decimal places as needed.) d. Find the 85 th percentile of blood pressure readings. The 85 th percentile of blood pressure readings is
Answers
The 85th percentile of blood pressure readings is approximately 137.64. a. To calculate the z-score for a blood pressure reading of 140, we can use the formula:
z = (x - μ) / σ
where x is the value (140 in this case), μ is the mean (121), and σ is the standard deviation (16).
Substituting the values into the formula:
z = (140 - 121) / 16
z ≈ 1.19 (rounded to two decimal places)
b. To find the proportion of Canadians with high blood pressure, we need to calculate the area under the normal distribution curve for values above 140. This can be done by finding the cumulative probability using the z-score.
Using a standard normal distribution table or a calculator, we can find that the cumulative probability corresponding to a z-score of 1.19 is approximately 0.881.
Therefore, the proportion of Canadians with high blood pressure is approximately 0.881 (rounded to four decimal places).
c. To find the proportion of Canadians with systolic blood pressure in the range from 100 to 140, we need to calculate the area under the normal distribution curve between these two values.
Using the z-scores corresponding to 100 and 140, we can find the cumulative probabilities for each value. The cumulative probability for a z-score of -1.25 (corresponding to 100) is approximately 0.105, and the cumulative probability for a z-score of 1.19 (corresponding to 140) is approximately 0.881 (as calculated in part b).
The proportion with systolic blood pressure between 100 and 140 is the difference between these two probabilities:
Proportion = 0.881 - 0.105 ≈ 0.776 (rounded to four decimal places)
d. The 85th percentile represents the value below which 85% of the blood pressure readings fall. To find the 85th percentile, we need to determine the z-score that corresponds to an area of 0.85 under the normal distribution curve.
Using a standard normal distribution table or a calculator, we can find that the z-score corresponding to an area of 0.85 is approximately 1.04.
To find the actual blood pressure reading at the 85th percentile, we can use the z-score formula:
x = μ + (z * σ)
Substituting the values:
x = 121 + (1.04 * 16)
x ≈ 137.64
Therefore, the 85th percentile of blood pressure readings is approximately 137.64.
To know more about blood pressure visit:
https://brainly.com/question/27876922
#SPJ11
the vsepr model is used mainly to multiple choice question. a) write resonance structures. b) determine molecular shape. c) measure intermolecular distances. d) determine ionic charge.
Answers
The VSEPR theory predicts the form of the molecule based on the bonding electron pairs and lone electron pairs around the core atom since molecular shape is dependent on the electrons surrounding the central atom.
How does the VSEPR theory describe the structure of molecules?Valence shell electron pair repulsion is the name given to the underlying concept in molecular structures (VSEPR). In essence, it states that because electron pairs are made up of negatively charged particles, they are attracted to one another and want to keep as far apart as possible.
Based on the number of valence shell electron bond pairs between the atoms in a molecule or ion, the valence shell electron pair repulsion (VSEPR) hypothesis is a model used to predict 3-D molecular shape.
Learn more about VSEPR theory refer
https://brainly.com/question/14225705
#SPJ4
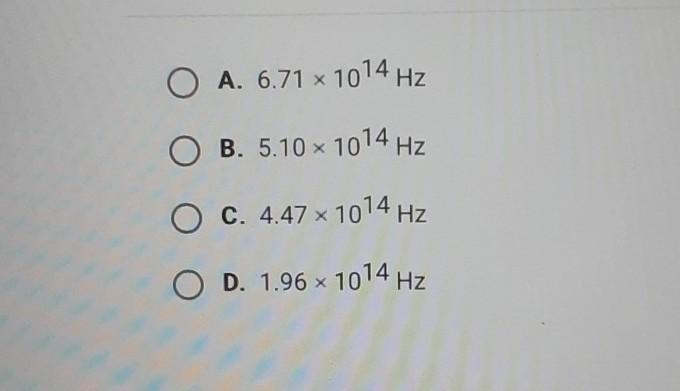
What is the frequency of a photon with an energy of 3.38 x 10-19 J?
Answers
\(\huge\boxed{5.1x\(10^{14}\)Hz}\)
_____________________________________DATA:E = \(3.38 x 10^{-19}\)
h = \(6.625x10^{-34}\)
f = ?
_____________________________________SOLUTION:Energy of Photon is given by,
E = hf
Rearrange the equation,
f = \(\frac{E}{h}\)
where,
E = energy
h = planck's constant
f = frequency
------------------------------------------------------------------------------------------------------------
Substitute the variable in the equation,
f = \(\frac{3.38x10^{-19}}{6.62x10^{-34}}\)
Simplify the equation,
f = 5.10 x 10^14 Hz
_____________________________________Best Regards,'Borz'
Answer:
b. 5.10 x 10^14 Hz
Explanation:
TRUE / FALSE. ind an appropriate parametrization for the given piecewise-smooth curve in double-struck r2, with the implied orientation.
Answers
The statement "In an appropriate parametrization for the given piecewise-smooth curve in double-struck \(r_2\), with the implied orientation" is false because it lacks clarity and specificity.
Parametrization in mathematics refers to expressing the coordinates of a curve in terms of a parameter such as time or arc length. It allows us to describe the position of points on the curve as a function of the parameter.
However, the given statement does not specify the appropriate parametrization for the curve, making it impossible to determine the validity of the statement. Moreover, the mention of "double-struck \(r_2\)" indicates the use of a two-dimensional Euclidean space, the statement is false.
To learn more about parametrization follow the link:
https://brainly.com/question/31461459
#SPJ4
An ethylene glycol solution contains 30.8 g of ethylene glycol (C2H6O2) in 96.6 mL of water. (Assume a density of 1.00 g/mL for water.) Determine the freezing point of the solution. Determine the boiling point of the solution
Answers
The freezing point of the solution is -11.8 °C.
The boiling point of the solution is 103.31 °C.
To determine the freezing point of the solution, we can use the equation:
ΔTf = Kf * m
where:
ΔTf is the freezing point depression,
Kf is the cryoscopic constant (for water, Kf = 1.86 °C/m),
m is the molality of the solution (moles of solute per kilogram of solvent).
First, let's calculate the molality (m) of the solution:
Molar mass of ethylene glycol (C2H6O2):
C = 12.01 g/mol
H = 1.01 g/mol (x 6) = 6.06 g/mol
O = 16.00 g/mol (x 2) = 32.00 g/mol
Total molar mass = 12.01 g/mol + 6.06 g/mol + 32.00 g/mol = 50.07 g/mol
Number of moles of ethylene glycol (C2H6O2) = mass / molar mass
Number of moles = 30.8 g / 50.07 g/mol = 0.615 mol
Mass of water = volume x density = 96.6 mL x 1.00 g/mL = 96.6 g
Now, let's calculate the molality:
Molality (m) = moles of solute / mass of solvent (in kg)
Molality = 0.615 mol / 0.0966 kg = 6.36 mol/kg
Now we can calculate the freezing point depression (ΔTf):
ΔTf = Kf * m
ΔTf = 1.86 °C/m * 6.36 mol/kg = 11.8 °C
To find the freezing point of the solution, subtract the freezing point depression from the freezing point of pure water (0 °C):
Freezing point = 0 °C - 11.8 °C = -11.8 °C
To determine the boiling point of the solution, we can use the equation:
ΔTb = Kb * m
where:
ΔTb is the boiling point elevation,
Kb is the ebullioscopic constant (for water, Kb = 0.52 °C/m),
m is the molality of the solution (same value as calculated before: 6.36 mol/kg).
ΔTb = 0.52 °C/m * 6.36 mol/kg = 3.31 °C
To find the boiling point of the solution, add the boiling point elevation to the boiling point of pure water (100 °C):
Boiling point = 100 °C + 3.31 °C = 103.31 °C
To know more about ethylene glycol
https://brainly.com/question/32452413
#SPJ11
How much energy does it take to raise the temperature of 80g of aluminium 15 degree Celsius
Answers
Answer :The specific heat of aluminium tells you the amount of energy needed to increase the temperature of 1 g of aluminium by 1∘C . You can thus say that in order to increase the temperature of 1 g of aluminium by 1∘C , you need to supply it with 0.214 cal of heat.
Explanation:
Answer:
\(\boxed {\boxed {\sf 1080 \ Joules}}\)
Explanation:
We are asked to calculate the energy needed to raise the temperature of 80 grams of aluminum by 15 degrees Celsius.
We are given the mass and change in temperature, so we will use the following formula:
\(q=mc\Delta T\)
The mass is 80 grams, the change in temperature is 15 degrees Celsius, and aluminum's specific heat capacity is 0.9 Joules per gram degree Celsius.
m=80 g ΔT= 15°C c= 0.9 J/g °CSubstitute the values into the formula.
\(q= (80 \ g)(0.9 \ J/g \textdegree C)(15 \textdegree C)\)
Multiply the first two numbers together. The units of grams will cancel out.
\(q= (80 \ g * 0.9 \ J/g \textdegree C)(15 \textdegree C)\)
\(q= (80 * 0.9 \ J/\textdegree C)(15 \textdegree C)\)
\(q= 72 \ J / \textdegree C (15 \textdegree C)\)
Multiply again. This time, the units of degrees Celsius cancel.
\(q= 72 \ J * 15\)
\(q= 1080 \ J\)
Raising the temperature of 80 grams of aluminum by 15 degrees Celsius requires 1080 Joules of energy.
Why does the amount of heat absorbed by an object depend on the type of surface?
Use these key words - radiation, emit, reflect, absorb, dark/light, waves
(We did an experiment seeing whether a black or silver can would absorb more radiation and the black can did)
Answers
The amount of heat absorbed by an object depends on the type of surface due to factors such as color, material, and emissivity. In your experiment, you observed that a black can absorbed more radiation than a silver one. This can be explained by two primary factors: absorption and emissivity.
Darker surfaces, like the black can, are more effective at absorbing heat because they have a higher absorption rate. Lighter surfaces, like the silver can, reflect more radiation, resulting in less heat absorption.
This difference in absorption rate is primarily due to the pigmentation of the materials, with darker colors being more absorptive and lighter colors being more reflective.
Emissivity is the measure of how effectively a surface emits thermal radiation. A surface with high emissivity will absorb and emit thermal radiation more efficiently than a surface with low emissivity.
Black surfaces typically have a higher emissivity compared to reflective surfaces like silver. In your experiment, the black can had a higher emissivity than the silver can, which means it absorbed and emitted thermal radiation more efficiently.
In summary, the amount of heat absorbed by an object depends on the type of surface due to differences in absorption and emissivity. Dark surfaces, like the black can in your experiment, have higher absorption rates and emissivity, which result in more efficient heat absorption and radiation compared to reflective surfaces like the silver can.
Know more about absorption here:
https://brainly.com/question/14609187
#SPJ11
5 points
14. What is the molality of a solution with 98.0 grams KOH in 425 grams
H20?*
A. 0.412 molal
B. 4.12 molal
C. 41.2 molal
D. 231 molal
Answers
Answer:
C 41.2
Explanation:
I took the test ez
brainliest and 5 star pls
PLEASE HELP ASAP! very much appreciated

Answers
Answer:
-Unknown
Explanation:
Questions is not seen properly bro
Fossil fuel is the best use of energy.
T
True
F
False
9 of 12
Answers
... I hope this helps
True
Answer:
True
Explanation:
Beacuse you use Fossil Fuels for gas and its oil as well
HELP ME THE WHOLE PAGE !!!! Someone help please

Answers
Answer:
In the image below
Explanation:
Hope this helps you :)

O2 transport in a person alternates between convection and diffusion. what best explains this pattern?
Answers
The pattern of alternating between convection and diffusion in O2 transport in a person can be attributed to the different methods by which O2 is transported in the body.
Convection refers to the movement of O2 through the bloodstream with the help of the circulatory system, while diffusion refers to the movement of O2 through the tissues and cells by means of diffusion gradients. During exercise or physical exertion, for example, the body may require more O2 than what can be supplied by convection alone. In these cases, diffusion plays a more significant role in O2 transport as it enables a greater amount of O2 to be delivered to the tissues and cells. Conversely, during rest or low levels of activity, convection may be the primary method of O2 transport as the body requires less oxygen. Therefore, the alternating pattern of convection and diffusion in O2 transport is a natural and necessary process that enables the body to efficiently deliver oxygen to where it is needed.
Learn more about diffusion here
https://brainly.com/question/30097487
#SPJ11
1. A gas is compressed and does P-V work on the system equal to 280 J. .At the same time, it releases 216 J of heat to the surroundings. What is the change in energy of the system?2. Which statement is false?i.Heat gives off by the system to the surrounding in the following reaction: CH4(g) + 2O2(g) -->CO2(g) + 2H2O(l) delta H = -890 kJ/molii.Heat is absorbed by the system from the surrounding in the following process: H2O(s) --> H2O(l) ?H = +6 kJ/moliii.Heat, q, is a state function.iv.Enthalpy, H, is a state function.3. Which statement is false associated with the following process? H2O(l) --> H2O(s) delta H = - 6 kJ/mol 6 kJ/mol of heat is released when water becomes ice.i.The surrounding gets warm as a result of this process.ii.The reverse process is endothermic.iii.6 kJ/mol of heat is to be absorbed when ice becomes water.iv.No false statement (i.e. all statements above are true.)4. A gas is compressed in a cylinder from a volume of 20 L to 2.0 L by a constant pressure of 10 atm. Calculate the amount of work done on the system. (1 atm L = 101.3 J)a. 1.01 x 104 Jb. -180 Jc. 1.81 x 104 Jd. -1.81 x 104 Je. 180 J
Answers
The correct answer is c. 1.81 x 10^4 J. Here positive sign denotes that work is done on system.
The solutions to different questions are as follows:
1. To find the change in energy of the system, you can use the formula:
ΔE = q + w, where ΔE is the change in energy, q is the heat released, and w is the work done on the system.
In this case, q = -216 J and w = 280 J.
Therefore, ΔE = (-216 J) + (280 J) = 64 J. So, the change in energy of the system is 64 J.
2. The false statement is iii. Heat, q, is a state function. Heat (q) is not a state function, as it depends on the path taken during a process not on the initial and final state. Enthalpy (H), on the other hand, is a state function.
3. The false statement associated with the process H2O(l) --> H2O(s) ΔH = -6 kJ/mol is i. The surrounding gets warm as a result of this process. When water becomes ice, heat is released from the system, but it will not cause the surroundings to get warm, as the released heat is being used to change the state of the substance.
4. To calculate the work done on the system, you can use the formula: w = -PΔV, where P is the constant pressure and ΔV is the change in volume.
In this case, P = 10 atm and ΔV = 20 L - 2.0 L = 18 L.
So, w = -10 atm * 18 L = -180 atm L. Since 1 atm L = 101.3 J, w = -180 atm L * 101.3 J/atm L = -1.81 x 10^4 J. The correct answer is c. 1.81 x 10^4 J. Here positive sign denotes that work is done on system.
To learn more about work done on/by the system, visit here:
https://brainly.com/question/20309171
#SPJ11