How many molecules of ethanol (C2H5OH) (the alcohol in alcoholic beverages) are present in 165mL of ethanol? The density of ethanol is 0.789 g/cm3.
Answers
There are 5.23 x 10²² molecules of ethanol present in 165mL of ethanol.
The density of ethanol is 0.789 g/cm³.A molecule is defined as the smallest particle in a chemical element or compound that has the chemical properties of that element or compound. This implies that all substances, including alcohol, are made up of molecules.
The first step in determining the number of molecules is to calculate the mass of ethanol present in 165 mL. The mass of ethanol can be calculated using the density formula:Mass = Density x VolumeTherefore, the mass of 165 mL of ethanol is:
Mass = 0.789 g/mL x 165 mL
= 130.4 g
To determine the number of ethanol molecules present, we must first determine the number of moles of ethanol in 130.4 g of ethanol. To determine the number of moles, we must divide the mass by the molecular weight of ethanol.
The molecular weight of ethanol (C₂H₅OH) is 46.07 g/mol.Moles of ethanol = Mass of ethanol / Molecular weight of ethanol= 130.4 g / 46.07 g/mol= 2.83 moles
Finally, we can use Avogadro's number to calculate the number of molecules in 2.83 moles of ethanol.Avogadro's number is 6.022 x 10²³ molecules/mol.
The number of molecules of ethanol present in 165 mL of ethanol is therefore:5.23 x 10²² molecules (to three significant figures).
To know more about Avogadro's number click on below link:
https://brainly.com/question/28812626#
#SPJ11
Related Questions
Letter D represents an energy level that holds electrons, what is another name for that ring, or energy level?
Answers
Answer:
Atomic orbital
Explanation:
Electrons are found around the atomic nucleus in three-dimensional regions.
The energy levels they are in can be named according to a numbering order such as 1, 2, 3, 4, ..., increasing in proportion to the distance to the nucleus. Each of these energy levels can be divided into sublevels s, p, d, f which are also called orbitals.
The difference between doing compositional stoichiometry and reaction stoichiometry problems is:
Answers
There are mass/mole ratios of elements in compounds in compositional stoichiometry questions and mass/mole ratios of compounds in balanced equations in reaction-based stoichiometry problems.
Composition stoichiometry relates to the atomic structure of a chemical compound, whereas reaction stoichiometry refers to the quantity of compounds consumed or created during a chemical reaction. This is the main distinction between composition and reaction stoichiometry.
Chemically speaking, the term "stoichiometry" refers to the quantitative information about a chemical substance or chemical reaction. When the information pertains to a chemical compound, it is referred to as composition stoichiometry; when it pertains to a chemical reaction, it is referred to as reaction stoichiometry.
Learn more about stoichiometry here: https://brainly.com/question/14935523
#SPJ4
A 1.00 L sample of a gas has a mass of 1.92 g at STP. What is the molar mass (molecular weight) of the gas
Answers
Answer:
The answer is below
Explanation:
Avogadro stated that "Equal volumes of all gases at the same temperature and pressure contain the same number of molecules". Hence at standard temperature and pressure 1 mole of a gas occupies 22.4 liters of volume.
1 mol = 22.4 liters
Given a gas with 1 L and mass of 1.92 g at STP.
number of moles = 1 L / (22.4 L / mol) = 0.0446 mol
The molar mass = mass of gas / number of moles
molar mass = 1.92 g / 0.0446 mol
molar mass = 43.008 g / mol
Given:
Mass = 1.92 gSample of gas = 1.00 LNow,
Number of moles will be:
= \(\frac{1 \ L}{22.4 \ L/mol}\)
= \(0.446 \ mol\)
hence,
The molar mass,
= \(\frac{Mass \ of \ gas}{Number \ of \ moles}\)
By putting the values,
= \(\frac{1.92}{0.446}\)
= \(43.008 \ g/mol\)
Thus the answer above is correct.
Learn more about molar mass here:
https://brainly.com/question/18619412
QUICK PLEASE HELP ME 30 POINTS RIGHT ANSERS ONLY :)
what term describe this particle model nh3, oh-, nh4+

Answers
Answer: Its a weak base
Explanation: Clicked on that and got the answer right. :)
The image that has been shown has helped us to know that the particles are weak bases. Option A
What is a weak base?
A chemical species or substance that has a restricted capacity to receive or interact with protons (H+ ions) in a solution is said to be a weak base. Weak bases only partially ionize or interact with water, in contrast to strong bases, which totally breakdown into ions in water and quickly take protons.
Compared to strong bases, weak bases have a lesser affinity for protons and fewer alkaline characteristics. They are frequently identified by the considerably lower concentration of hydroxide ions (OH-) in a solution and their imperfect dissociation equilibrium.
Learn more about weak base:https://brainly.com/question/28246086
#SPJ1
A 0.25 M solution of a salt NaA has pH 9.29. What is the value of Ka for the parent acid HA?
Answers
The Ka value for the parent acid of Ha is \(Ka = 6.576 \times 10^{-6}\).
Overall equation for this reaction can be given as :
Na+ (aq) + A-(aq) + H2O(l) → Ņa+(aq) + HA(aq) + OH-(aq)
Net ionic equation will be :
\(A^{-}(aq) + H_{2}O(l) \rightarrow HA(aq) + OH^{-}(aq)\\\\K_{b} = \frac{[HA][OH^{-}]}{[A^{-}]}\)
Given, pH = 9.29
The pH of the solution is the negative logarithm of hydrogen ion concentration in aqueous solution.
So, pOH = 14 - 9.29 = 4.71
So, \([OH^-] = 10-4.71 = 1.95 \times 10^-{5}\)
M = [HA]
\(K_{b} = \frac{[HA][OH^{-}]}{[A^{-}]}or, K_{b} = \frac{[1.95 \times 10^{-5}]^{2}}{[0.25]}or, K_{b} = 1.521 \times 10^{-9}\)
Now, we know that;
\(K_{a} \times K_{b} = 10^{-14}or, K_{a} = \frac{10^{-14}}{1.521 \times 10^{-9}}or,\mathbf{ K_{a} = 6.576 \times 10^{-6}}\)
Therefore, the value of \(Ka = 6.576 \times 10^{-6}\).
To learn more about pH check the link below-
https://brainly.com/question/172153
#SPJ4
Name the following compounds:

Answers
The correct name of the given compound is 2 methyl butane, and 2 - methyl-2- pentene.
The suffix '-ane' is used for alkanes, '-ene' for alkenes, and 'yne' for alkynes. For instance, C₂H₆ is referred to as ethane, C₂H₄ is referred to as ethene, and C₂H₂ is referred to as ethyne.
In order to get to the double- or triple-bonded carbon atom first, the parent chain is numbered.
In hydrocarbons, the suffix -ene is used in place of -ane to denote double bonds. The suffix is expanded to add a prefix that denotes the number of double bonds present if there are more than one double bond.
Learn more about butane, here:
https://brainly.com/question/30765150
#SPJ1
p draw the particle by placing atoms on the grid and connecting them with bonds. include all lone pairs of electrons and nonbonding electrons.
Answers
When a sulphur atom takes two electrons, the S2- ion is created.
What are Electrons?
Electrons are negatively-charged subatomic particles found in atoms. They are the smallest particles in an atom and are responsible for carrying electrical charge and energy. They exist in a cloud of energy around the nucleus of an atom, and can move between atoms.
Sulphur has six electrons in its outermost shell by default, but it can accept two more to complete its octet and create the sulphide (S^2-) ion.
What is Sulphur?
Sulphur is a non-metallic chemical element with the symbol S and atomic number 16. It is abundant, multivalent and also non-metallic. Under normal conditions, sulphur atoms form cyclic octatomic molecules with a chemical formula S8.
To know more about Electrons,
https://brainly.com/question/28337734
#SPJ4
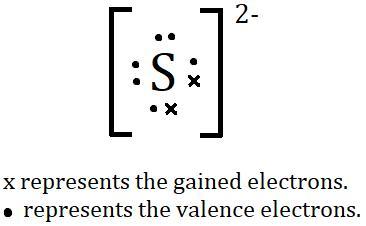
In the isothermal reversible compression of 1.77 mmol of a perfect gas at 273k, the volume of the gas is reduced to 0.224l of its initial value. calculate the work for the process.
Answers
To calculate the work for the isothermal reversible compression of a perfect gas, we are given the initial amount of gas (1.77 mmol), the initial temperature (273 K), and the final volume (0.224 L) in relation to its initial volume.
With these values, we can determine the work using the formula for work in an isothermal reversible process.
The work done in an isothermal reversible process can be calculated using the formula:
Work = -nRT ln(Vf/Vi)
where:
- n is the number of moles of gas
- R is the gas constant
- T is the temperature in Kelvin
- Vf is the final volume
- Vi is the initial volume
Substituting the given values into the formula, we have:
- n = 1.77 mmol = 0.00177 mol
- R = ideal gas constant (8.314 J/(mol·K))
- T = 273 K
- Vf = 0.224 L (final volume)
- Vi = initial volume
Now let's substitute the values and calculate the work:
Work = - (0.00177 mol) * (8.314 J/(mol·K)) * 273 K * ln(0.224 L / Vi)
Please note that the exact value of the work will depend on the specific value of the initial volume (Vi). By substituting the given values into the formula and performing the necessary calculations, you can determine the work for the isothermal reversible compression process.
To know more about isothermal , click here-
brainly.in/question/6527631
#SPJ11
___ Uses moving air and doesn't pollute the atmosphere.____ uses dead plant matter and burning it releases carbon emissions into the atmosphere.
1. 2.
a. Biomass Energy a. Biomass Energy
b. Solar energy b. Solar energy
c. Hydropower Energy c. Hydropower energy
d. Wind Energy d. Wind Energy
Answers
Answer: 1 d. Wind Energy 2. a. Biomass Energy
Explanation:
The use of the moving air has been found to use wind energy, and the dead plants burning is formed with the biomass energy.
What is energy?The energy has been given as the required form to perform a task. The energy can be generated with the pressure and the force of various matters.
Wind energy is produced with the use of the moving air and has been a clean form of energy, i.e. not pollute the region.
The biomass energy has been produced with the use of the dead plants and the matter and releases carbon dioxide.
Learn more about energy, here:
https://brainly.com/question/8630757
What is the difference between a number and a measurement ?
Answers
Answer:An exact number has absolutely no uncertainty in it. Exact numbers cannot be simplified and have an infinite number of significant figures. Measured numbers have a limited number of significant figures.
Explanation:
When referring to bacteria cells, a plasmid
is not naturally occuring.
does not code for proteins.
is the large bacterial chromosome contained in the nucleoid.
is composed of double-stranded RNA.
is a small, circular double-stranded DNA molecule that replicates autonomously.
Answers
Answer:
is a small, circular double-stranded DNA molecule that replicates autonomously.
Explanation:
hope this helps
0.22g of carbon dioxide are dissolved in 400 cm3 of pure water.
Calculate the concentration in mol/dm3 of the
solution produced.
Answers
Answer:
0.0125mol/dm³
Explanation:
Given parameters:
Mass of carbon dioxide = 0.22g
Volume of water = 400cm³
Unknown:
Concentration in mol/dm³ = ?
Solution:
Concentration is the amount of solute dissolved in a solvent.
The formula is expressed as;
Concentration = \(\frac{number of moles}{volume}\)
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of CO₂ = 12 + 3(16) = 44g/mol
Number of moles = \(\frac{0.22}{44}\) = 0.0005mol
Now,
1000cm³ = 1dm³
400cm³ = \(\frac{400}{1000}\) = 0.4dm³
Insert the parameters and solve;
Concentration = \(\frac{0.005}{0.4}\) = 0.0125mol/dm³
An aluminium soft drink can is approximately 0. 55 moles of aluminium. How many aluminium atoms are used in manufacturing each soft drink can?
Answers
To determine the number of aluminum atoms used in manufacturing each soft drink can, we need to multiply the number of moles of aluminum (0.55 moles) by Avogadro's number (6.022 × 10^23 atoms/mol). Therefore, each soft drink can contains approximately 3.31 × 10^23 aluminum atoms.
To determine the number of aluminum atoms used in manufacturing each soft drink can, we need to know the Avogadro's number, which represents the number of atoms or molecules in one mole of a substance.
Avogadro's number (NA) is approximately 6.022 × 10^23 atoms/mol.
Given:
Moles of aluminum (Al) = 0.55 moles
To find the number of aluminum atoms, we can use the relationship between moles and atoms:
Number of atoms = Moles × Avogadro's number
Number of atoms = 0.55 moles × (6.022 × 10^23 atoms/mol)
Number of atoms ≈ 3.3121 × 10^23 atoms
Therefore, approximately 3.3121 × 10^23 aluminum atoms are used in manufacturing each soft drink can.
To know more about Avogadro's number click this link -
brainly.com/question/28812626
#SPJ11
You titrate 25.00 ml of 0.1894 M acetic acid with 0.2006 M NaOH. How many ml of NaOH (to four significant figures) will be required at the equivalence point?
Answers
Answer:
23.60 mL NaOH
Explanation:
The reaction is CH3COOH + OH- --> CH3COO+ + H2O
Since the reaction is one-to-one, we can use M1V1 = M2V2.
M1 = 0.1894 M CH3COOH
V1 = 25.00 mL CH3COOH
M2 = 0.2006 M NaOH
V2 = ?
Solve for V2 --> V2 = M1V1/M2
V2 = (0.1894 M)(25.00 mL) / (0.2006 M) = 23.60 mL NaOH
Which statement is true about electronegativity?
A. Electronegativity generally decreases from left to right across a period.
B. Electronegativity is the ability of an anion to attract another anion.
C. Electronegativity generally is higher for metals than for nonmetals.
D. Electronegativity generally increases as you go from bottom to top in a group.
Answers
Answer:
electronegativity generally increase as you go from bottom to top group
Ethane, C2H6, burns in air according to the equation
2 C2H6(g) + 7 O2(g) 4 CO2(s) + 6 H2O(g)
How many liters of O2 are required for complete reaction with 4.0 L of C2H6?
Answers
Explanation: The balanced chemical equation for the combustion of ethane (C2H6) is:
2 C2H6(g) + 7 O2(g) → 4 CO2(s) + 6 H2O(g)
According to the equation, 7 liters of O2 are required for a complete reaction with 4.0 L of C2H61.
consider a bimolecular reaction in the gas phase. which one of the following changes in condition will not cause an increase in the rate of the reaction?
Answers
Out of the given options, increasing the volume at constant temperature will not cause an increase in the rate of the bimolecular gas-phase reaction.
This is because the rate of the reaction depends on the concentration of reactant molecules. When the volume is increased at constant temperature, the concentration of reactant molecules decreases as they are more spread out over a larger volume. As a result, the rate of the reaction decreases.
On the other hand, adding a catalyst or increasing the temperature at constant volume will increase the rate of the reaction. A catalyst provides an alternative pathway for the reaction with a lower activation energy, making it easier for the reactant molecules to react.
This leads to an increase in the rate of the reaction. Similarly, increasing the temperature increases the kinetic energy of the reactant molecules, leading to more collisions and therefore, an increased rate of reaction.
Overall, it is important to consider the effect of changing conditions on the concentration of reactant molecules when predicting the rate of a reaction.
For more such questions on gas-phase reaction, click on:
https://brainly.com/question/30316915
#SPJ11
The probable question may be:
consider a bimolecular reaction in the gas phase. which one of the following changes in condition will not cause an increase in the rate of the reaction?
add a catalyst increase the temperature at constant volume Increase the volume at constant temperature All of the above will increase the rate of reaction
a hypothetical element x has 3 naturally occurring isotopes; x-40, x-41 and x-42. their abundances are 72.0%, 9.00%, and 19.0% respectively. what is the atomic mass of x?
Answers
Therefore, the atomic mass of element x is approximately 40.47. It is important to note that this is a hypothetical element and may not actually exist in nature.
To find the atomic mass of element x, we need to first calculate the weighted average of the atomic masses of its isotopes, taking into account their respective abundances. We can use the following formula:
atomic mass of x = (% abundance of x-40 × atomic mass of x-40) + (% abundance of x-41 × atomic mass of x-41) + (% abundance of x-42 × atomic mass of x-42)
Plugging in the given values, we get:
atomic mass of x = (0.720 × 40) + (0.090 × 41) + (0.190 × 42)
atomic mass of x = 28.8 + 3.69 + 7.98
atomic mass of x = 40.47
To know more about atomic mass visit:
https://brainly.com/question/17067547
#SPJ11
What is the reaction to . Na2SO3 + 2 HC2H3O2 → H2SO3 + 2 NaC2H3O2
Answers
explain why you would expect the molar heat capacity at constant pressure of an ideal gas to be larger than the molar heat capacity at constant volume.
Answers
The thermal capacity under a certain pressure Since the substance expands and produces energy when heat is applied at a constant pressure, CP is greater than the heat capacity at constant volume CV.
Why does the first law of thermodynamics demonstrate that CP Cv R and that specific heat at constant pressure is greater than that at constant volume?In the first instance, the temperature of the gas must be raised with greater heat. Since it requires more energy to raise the temperature by one unit in the former situation, CP, or specific heat at constant pressure, is greater than CV, or specific heat at constant volume.
To know more about heat capacity visit:-
https://brainly.com/question/28302909
#SPJ4
can someone please help me i need help asap please

Answers
2) Summary: main points and highlights from the body paragraphs
3) Significance: the relevance and implications of the essay's findings
Help! Fast please lol

Answers
Answer:
Aluminum
Explanation:
The element with the given shell notation of electronic configuration is aluminum.
The given configuration is :
[Ne] 3s² 3p¹
To find this chemical specie, we need to know the number of electrons it contains first;
The superscript is an indicator of the number of electrons in each of the subshell;
[Ne] = 1s² 2s² 2p⁶ = 2 + 2 + 6 = 10
3s² 3p¹ = 2 + 1 = 3
Total number of electrons = 10 + 3 = 13 electrons
The element with 13 electrons is aluminum.
Which statement best summarizes how viruses cause disease? A. Viruses absorb nutrients from another organism's body. B. Viruses use living cells to make new viruses. C. Viruses reproduce best in warm, moist environments. D. Viruses use cell parts for nutrients.
Answers
Answer:
its not d its b i took the quiz
Explanation:
reactions that tend to go on their own, releasing energy, are called:
Answers
Reactions that tend to go on their own, releasing energy, are called exergonic reactions.
In an exergonic reaction, the energy of the products is lower than the energy of the reactants. As a result, energy is released during the reaction. This energy can take various forms, such as heat, light, or the energy used to perform work.
Exergonic reactions are spontaneous and do not require an external energy source to proceed. The energy released during these reactions is often utilized by the surrounding environment or used to drive other cellular processes.
An example of an exergonic reaction is the combustion of fuel, such as gasoline. When gasoline reacts with oxygen in the presence of heat or a spark, it undergoes an exergonic reaction, releasing energy in the form of heat, light, and mechanical work.
In biological systems, cellular respiration is an example of an exergonic reaction. During cellular respiration, the breakdown of glucose in the presence of oxygen releases energy that is used by cells to perform various functions, including muscle contraction, active transport, and synthesis of molecules like ATP.
Overall, exergonic reactions play a fundamental role in energy transfer and metabolism, driving many essential processes in both biological and non-biological systems.
Here you can learn more about exergonic reaction
https://brainly.com/question/30800156#
#SPJ11
Vaporization is the reverse of condensation. Select one: True False
Answers
Answer:
True
Explanation:
What are 4 examples of amorphous solids?
Answers
Four examples of amorphous solids are: Glass, Rubber, Asphalt, and Amorphous metals.
Amorphous solids are solids that lack a long-range ordered structure and have a disordered arrangement of atoms or molecules.
Glass: Glass is a non-crystalline solid that is made by cooling a melt or solution so rapidly that the atoms or molecules do not have time to arrange themselves into a crystalline structure.
Rubber: Natural rubber and synthetic rubber are both examples of amorphous solids. Rubber is made up of long polymer chains that are tangled and disordered, giving it its characteristic elasticity and flexibility.
Asphalt: Asphalt is a mixture of bitumen and mineral aggregates that is used as a paving material for roads, parking lots, and other surfaces. Asphalt is an amorphous solid because the bitumen molecules have a disordered arrangement.
Amorphous metals: Amorphous metals, also known as metallic glasses, are a class of metals that have a disordered atomic structure. Amorphous metals are made by cooling a liquid metal at a rate of millions of degrees per second, which prevents the atoms from arranging themselves into a crystalline structure.
To know more about amorphous solids here
https://brainly.com/question/28274778
#SPJ4
what is the cone of uncertainty
Answers
Answer:
The cone of uncertainty represents the evolution of the amount of best-case uncertainty during a project. Predicting the success of a project is extremely difficult. Many factors can influence a company's estimate and contribute to project uncertainties. I hope that this helps you UwU
The last point of comparison is to look at oxygen isotope data for this time interval. It is a tricky business to estimate temperature from isotope values from so long ago because quite a bit can change about both oceans and organisms over such a long time. People who study these super ancient isotope records feel that some corrections are needed to account for these changes. I am mostly (but not 100%) convinced that they are justified. If you want, I can give you the paper where they explain the basis for the corrections, but it involves some serious chemistry.
C) Chemical proxies for temperature
In the graph below, the uncorrected values are shown with the blue line. The corrected values are shown with either the red or the dashed black line (there are two different ways of doing the corrections). They are roughly the same, so let's use the dashed black line as it is a little easier to see. 5) How well do the uncorrected dOvalues correspond with CO, levels? What about the corrected dashed black line? Sometimes we are looking at whether something is increasing or decreasing. In this case I want you to also look at where the values lie relative to today (the horizontal dashed line at 0°C)
6) Does this argue for or against the notion that CO2 concentration is one of
Answers
We can analyze the relationship between the uncorrected dO (oxygen isotope) values and CO2 levels, as well as the corrected dashed black line values.
In terms of the uncorrected dO values, it is unclear how well they correspond with CO2 levels since the specific correlation or trend is not mentioned. Without further details or data, we cannot determine the exact relationship between the uncorrected dO values and CO2 levels.
However, regarding the corrected dashed black line values, we can observe their alignment with the horizontal dashed line at 0°C, which represents today's temperature. By assessing where the corrected values lie relative to this line, we can gain insights into temperature changes over time.
Based on the information provided, we cannot definitively conclude whether this argues for or against the notion that CO2 concentration is one of the main drivers of climate change. The given context focuses on comparing the dO values with CO2 levels and temperature, without explicitly addressing the relationship between CO2 concentration and climate change. To draw conclusions about the impact of CO2 concentration on climate change, further analysis and information about the specific trends and patterns are required.
Overall, without additional data and details, it is challenging to determine the exact correspondence between the uncorrected dO values and CO2 levels, as well as the implications for the role of CO2 concentration in climate change. Further examination of the provided paper and relevant scientific literature would provide a more comprehensive understanding of the topic.
Learn more about dashed black line values from below link
https://brainly.com/question/33540001
#SPJ11
Determine the molar mass of octane, C 8, H 18
Answers
Answer:
The molar mass of C8 H18 is 114.22852 g/mol.
Explanation:
The molecular formula C8H18 (molar mass: 114.23 g/mol) may refer to: Octane (n-octane)
If a student has 125 mL of a 4.00 M CuSO4 solution and needs a 1.50 M solution, what volume do they need to dilute it to?
Answers
Answer:
333.3mL
Explanation:
Using the formula as follows:
C1V1 = C2V2
Where;
C1 = initial concentration (M)
C2 = final concentration (M)
V1 = initial volume (mL)
V2 = final volume (mL)
According to the information provided in this question,
C1 = 4.00M
C2 = 1.50M
V1 = 125mL
V2 = ?
Using C1V1 = C2V2
4 × 125 = 1.5 × V2
500 = 1.5V2
V2 = 500/1.5
V2 = 333.3mL
Therefore, the CuSO4 solution needs to be diluted to 333.3mL to make 1.50 M solution.