How many moles of HCl need to be added to 150.0 mL of 0.50 M NaZ to have a solution with a pH of 6.50? (K a of HZ is 2.3 x 10 -5 .) Assume negligible volume of the HCl.mol
Answers
To create a solution with a pH of 6.50, 150.0 mL of 0.50 M NaZ needs to have 0.071 moles of HCl added to it.
To calculate the moles of HCl needed, follow these steps:
1. Calculate the moles of NaZ in the solution:
moles NaZ = (0.50 mol/L) * (150.0 mL) / (1000 mL/L) = 0.075 mol
2. Determine the concentration of HZ and Z- after HCl is added:
pH = pKa + log([Z-]/[HZ])
6.50 = -log(2.3 x \(10^{-5\)) + log([Z-]/[HZ])
3. Solve for the ratio [Z-]/[HZ]:
0.075 mol NaZ = [Z-] + [HZ]
[Z-]/[HZ] = \(10^{(6.50 - (-log(2.3)*10^{-5} )))}\) = \(10^{(6.50 - 4.64)\) = \(10^{1.86\)
4. Use the ratio to find [HZ] and [Z-]:
[HZ] = 0.075 mol / (1 + \(10^{1.86\) )
[HZ] ≈ 0.00366 mol
5. Calculate the moles of HCl needed to convert Z- to HZ:
moles HCl = moles NaZ - moles HZ = 0.075 - 0.00366 ≈ 0.071 mol
Learn more about moles here:
https://brainly.com/question/28239680
#SPJ11
Related Questions
Plzz giving brainlist to first answer!Plss fill in the blanks!!ASAP.Modeling Our Solar System
The planets follow these ____________ paths as they travel around the Sun. The Sun’s ______________
makes the planets move in this way. It pulls on the planets, keeping them in motion in their ____________.
Modeling is a way to ________________ or _____________ a particular idea or
concept.
Models do have some ______________________.
Often a model is made with certain _______________________.
A model of the solar system may contain all of the planets and the Sun, but it may not include the planets’
individual moons or every __________ and ________________ in the solar system.
need to be updated regularly with new information
Example: Older models of the solar system may show ________ planets, which we now know is inaccurate.
can draw by hand or even use computer software
Creating a Model
Question 1
Part A
The table shows the diameters of the planets in our solar system.
1. Find the scale (ratio) between Jupiter and the basketball.
2. Then use this ratio to find the scaled diameter of the other planets.
3. Enter these numbers into the table.
4. Finally, choose a real-world spherical or nearly spherical object that matches the scaled diameter of eac
Answers
Answer:
The planets follow these orbit paths as they travel around the Sun. The Sun’s gravity
makes the planets move in this way. It pulls on the planets, keeping them in motion in their orbit.
Modeling is a way to see ideas or have a particular idea or
concept.
Models do have some lines .
Often a model is made with certain things.
A model of the solar system may contain all of the planets and the Sun, but it may not include the planets’
individual moons or every planit and rock in the solar system.
Explanation: if you think about it and and look at pics it is easy
hope this helps
4. Lithium + tin(IV) sulfite ® lithium sulfite + tin-
Answers
2Li2S + Sn(SO4)2 → 2Li2(SO4) + SnS2 is Balanced chemical equation.
What is Balanced chemical equation?
A balanced chemical equation is a chemical reaction that has equal numbers of moles of chemical substances both in the reactant and the product side.
A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides.
In a balanced chemical equation, the mass and the change are both equal.
From the missing image that is attached below, we can see that the picture depicts that we have:
The reactant side:
3 molecules of CH₄ and 3 molecules of N₂Cl₄
The product side:
3 molecules of CCl₄, 3 molecules of N₂ and 6 molecules of H₂
Therefore, the balanced chemical equation for the reaction is:
3CH₄ + 3 N₂Cl₄ → 3 CCl₄ + 3N₂ + 6H₂
Learn more about Balanced chemical equation from given link
https://brainly.com/question/26694427
#SPJ4
Select the correct locations on the image.
Identify the arrows that show removal of thermal energy when matter changes state.

Answers
The backward arrows show removal of thermal energy when matter changes state.
What is thermal energy?Thermal energy or heat energy is energy due to temperature differences.
Heat energy flows from hotter to colder bodies.
Addition or removal of heat results in change of state of substances.
Removal of heat energy results in condensation or freezing.
Therefore, the backward arrows show removal of thermal energy when matter changes state.
Learn more about thermal energy at: https://brainly.com/question/19666326
#SPJ1
Answer:
Bottom 2 arrows
Explanation:
got it right took the test
the first ionization energy of selenium is 941 kj/mol. calculate the maximum wavelength of a photon that can ionize se.
Answers
The greatest wavelength at which a photon may get ionized is 2.294 107 nm. When an atom or molecule acquires or eliminates an energy, the activity of ionization creates ions.
The ionization energy rule is what?
Upon that annual chart, that the very first oxidation number typically climbs towards left to right for each period. The higher nuclear charge results in a closer bond between the outermost electron and the nucleus.
How does ionization work?
Any process by which electrically neutral atoms or molecules are changed into electrically charged atoms or molecules (ions) by the gain or loss of electrons is referred to as ionization in chemistry and physics.
Briefing:
ionization energy= 941kj/mol
ionization energy= 941×1/6.022×10²³
=15.62×10⁻²²kj/atom
E=h∨
v=15.62×10⁻²²×10³/6.626×10⁻³⁴
=1.3075×10¹⁰s⁻¹
λν=c
λ=3×10⁸/1.3075×10¹⁰
=2.294×10⁻²m
To know more about ionization energy visit:
https://brainly.com/question/28385102
#SPJ4
In a chemistry experiment, 50.00 ml 50.00 ml of 2.0 m 2.0 m h n o 3 hno3 is titrated with 0.50 m 0.50 m n a o h naoh. what volume of n a o h naoh, in ml ml, is required to reach the equivalence point?
Answers
200ml of NaOH is required to reach the equivalence point.
Titration is a standard quantitative chemical analysis laboratory method for determining the concentration of a specified analyte. The titrant or titrator is a reagent that is produced as a standard solution of known concentration and volume. Titration is an essential technique in analytical chemistry, and it is also known as volumetric analysis.
A chemical reaction's equivalence point, also known as the stoichiometric point, is the point at which chemically equivalent quantities of reactants have been combined. The equivalence point for an acid-base reaction is the point at which the moles of acid and base would neutralise each other according to the chemical reaction.
To learn more about equivalence point and titration, here
https://brainly.com/question/14245137
#SPJ4
What is the approximate pH of an acetate buffer with equimolar concentrations of acetic acid and sodium acetate? A. 9. B. 7. C. 5. D. 1. 5.
Answers
The approximate pH of an acetate buffer with equimolar concentrations of acetic acid and sodium acetate is 5.
Let's understand this in detail:
What is a buffer solution?
A buffer solution is an aqueous solution that has the ability to maintain a constant pH level. The buffer's most significant property is the ability to resist changes in pH when small quantities of acids or bases are added.
The buffer solution comprises a weak acid and its conjugate base and a weak base and its conjugate acid.
The Henderson-Hasselbalch equation is used to calculate the pH of a buffer:
Henderson-Hasselbalch equation
pH = pKa + log [A-]/[HA]Where pKa is the acid dissociation constant,
[A-] is the concentration of the conjugate base, and [HA] is the weak acid concentration.
The pKa value for acetic acid is 4.76. Since the concentrations of acetic acid and sodium acetate are equimolar, their concentrations are identical. Therefore, the concentration of the conjugate base is the same as the concentration of the weak acid.
Using the Henderson-Hasselbalch equation,
pH = 4.76 + log [A-]/[HA]pH = 4.76 + log 1pH = 4.76 + 0pH = 4.76
Therefore, the approximate pH of an acetate buffer with equimolar concentrations of acetic acid and sodium acetate is 5.
#SPJ11
Learn more about buffers: Describe the possible components of a buffer solution. https://brainly.com/question/8676275
Can you explain by illustration how the water changes from gas to liquid
Answers
The water changes from the gas to the liquid through the process of the condensation.
The Condensation is the process where the water vapor becomes the liquid. It is the reverse of the evaporation, where the liquid water becomes the vapor. The Condensation happens one of the two ways: The first is either the air will cooled to its the dew point or it will becomes so saturated with the water vapor that it cannot be hold any of the more water.
Thus, the condensation is the process in which the water changes from the gas phase to the liquid phase.
To learn more about condensation here
https://brainly.com/question/21566464
#SPJ4
7. R, the gas constant is equal to these three values, include units;
Answers
R, the gas constant is equal to these three values, volume, temperature, pressure and number of moles.
Depending on the other units used in the equation, different units are used for the gas constant. The Gas Constant's Value The units used for pressure, volume, and temperature have an impact on the value of the gas constant "R". These were typical gas constant values prior to 2019. R = 8.3145 J/mol K R = 8.2057 m 3 atm/mol K R = 0.0821 litre atm/mol K. Work per degree every mole is what R means physically. Any system of units for measuring labour or energy, such as joules, or for measuring temperature at an absolute scale, like as kelvin or rankine, may be used to express it.
To know more about gas constant, here:
https://brainly.com/question/14279790
#SPJ1
24.1% of all the isotopes of an element have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. How many protons
does this element have? (hint: Find the average atomic mass)
3
34
33
no way to know
Answers
The number of protons in the element is 33.
From the information provided we can find the relative atomic mass of the element as follows;
RAM = (0.241 × 75.23) + (0.487 × 74.61) + (0.272 × 75.20)
RAM = 18.13 + 36.34 + 20.45
RAM = 74.92 amu
The element with relative atomic mass of 74.92 amu is Arsenic.
The atomic number of arsenic is 33. This is the number of protons in its nucleus. Therefore, the number of protons in the element is 33.
Learn more:https://brainly.com/question/1371394
How many grams is 0.02 moles of CuCl2
Answers
Answer:
2.69g
Explanation:
The molar masses of
Cu = 63.5
Cl = 35.5
So the molar mass of \(CuCl_{2}\) is 63.5 + (35.5 x 2) = 134.5
134.5 x 0.02 = 2.69g
The normal boiling and freezing points of argon are 87.3 K and 84.0 K. The triple point is at 82.7 K and 0.68 atmosphere. Use the data above to draw a phase diagram for argon. In the diagram, label the spots where the solid, liquid, and gas phases are stable as well as show the position of the normal boiling point.
Answers
INFORMATION:
We know that:
- The normal boiling and freezing points of argon are 87.3 K and 84.0 K
- The triple point is at 82.7 K and 0.68 atmosphere.
And we must draw a phase diagram for argon using the data
STEP BY STEP EXPLANATION:
To draw the diagram, we must graph first the triple point since it is the common point for the three states of the argon
Then, we need to use that the normal boiling and freezing points of argon are 87.3 K and 84.0 K to divide he states of the argon in the graph
Finally, we need to show the position of the normal boiling point
So, we obtain the next graph
ANSWER:


Which BEST describes why fossil fuels are most often used for energy in the United States?
Answers
Answer:
fossil fuil is way easer to burn
Explanation:
also transport good luck!
Which of the following is a true statement? Select 2.
Gas particles are much smaller than the distances between them
Gas particles are the same size as the distances between them
Gas particles are much larger than the distances between them
The volume of a gas is mostly tightly packed particles.
The volume of a gas is mostly empty space.
The volume of a gas is equally empty space and tightly packed particles.
Answers
How many Hydrogen moles needed to get the energy to equal 1 mol of gasoline?
Answers
Answer:
1 grams Hydrogen is equal to 0.992122546977 mole.
Explanation:
Hydrogen is equal to 0.992122546977 mole.
which ph value is consistent with the indicator results
Answers
The color of the indicator will correspond to the pH values of either acidic or basic substances.
What are pH values and Indications?pH values are values which are obtained from taking the negative logarithm to base ten of the hydrogen ions concentration of a substance.
pH values of acidic substances are less than 7 while the pH of basic or alkaline substances are greater than 7.
An indicator is an organic dye which changes colour according to the pH of a substance.
Examples of indicators are methyl orange and phenolphthalein.
Therefore, the color of the indicator will correspond to pglh values of either acidic or basic substances.
Learn more about indicators at: https://brainly.com/question/1918667
#SPJ1
If 46g of HC4 react with 32g of O2
CH4+2O2=CO3+2H2O
Answers
The given chemical equation can be balanced as: CH4 + 2O2 → CO2 + 2H2OTherefore, the balanced chemical equation for the reaction between 46 g of CH4 and 32 g of O2 is:CH4 + 2O2 → CO2 + 2H2OMoles of CH4 = (46 g)/(16.04 g/mol) = 2.87 molMoles of O2 = (32 g)/(32 g/mol) = 1 mol.
Moles of CH4 = (46 g)/(16.04 g/mol) = 2.87 molMoles of O2 = (32 g)/(32 g/mol) = 1 molFor the given reaction, one mole of CH4 reacts with 2 moles of O2. Therefore, 2.87 moles of CH4 would react with 2 × 2.87 = 5.74 moles of O2. As we can see from the given values, only 1 mole of O2 is present. Hence, O2 is the limiting reactant.The balanced chemical equation for the reaction between 46 g of CH4 and 32 g of O2 is:CH4 + 2O2 → CO2 + 2H2OOn
The basis of the balanced chemical equation, 2 moles of O2 is required for one mole of CH4. Here, the moles of O2 is lesser than 2 times the moles of CH4, which means O2 is the limiting reactant and CH4 is the excess reactant. Hence, 32 g of O2 will react completely with 1 mole of CH4, and 14.14 g of CO2 and 4 g of H2O will be produced as per the stoichiometry.
To know more about chemical equation visit:
https://brainly.com/question/28792948
#SPJ11
Many watches are powered by small, flat batteries called button cells. One common type of button cell contains the metal zinc. When zinc in the battery combines with oxygen in the air, zinc oxide forms. This process generates the electricity that powers the watch. is this a product or reactant
Answers
The electricity generated that powers the watch by zinc in the battery combines with oxygen in the air, zinc oxide forms reaction is a product.
Zinc-air batteryIn zinc -air battery oxidation of zinc to zinc oxide in presence of oxygen from air takes place. These batteries are cost-effective and contain more density of energy as compared to others. When compared with lithium batteries these are with more capacity, environmental safe, cost-effective and easy to produce these are widely used. Metal-air batteries fueled by the oxidation of zinc with oxygen from the air include zinc-air batteries (non-rechargeable) and zinc-air fuel cells (mechanically rechargeable). These batteries are produced at comparatively low costs and have great energy densities.Effective anode materials must be developed for next-generation lithium-ion batteries (LIBs). Due to a number of intriguing characteristics, including its high theoretical capacity, simplicity in synthesis, environmental friendliness, and low cost, zinc oxide (ZnO) has been regarded as a useful material.In addition, compared to the majority of primary batteries, zinc-air batteries offer a high volumetric energy density. Such batteries have a number of drawbacks, including a reliance on ambient conditions, a propensity to dry up when exposed to air, flooding potential, a finite output, and a brief active life.For more information on zinc batteries kindly visit to
https://brainly.com/question/15308599
#SPJ1
What’s the answer to this?
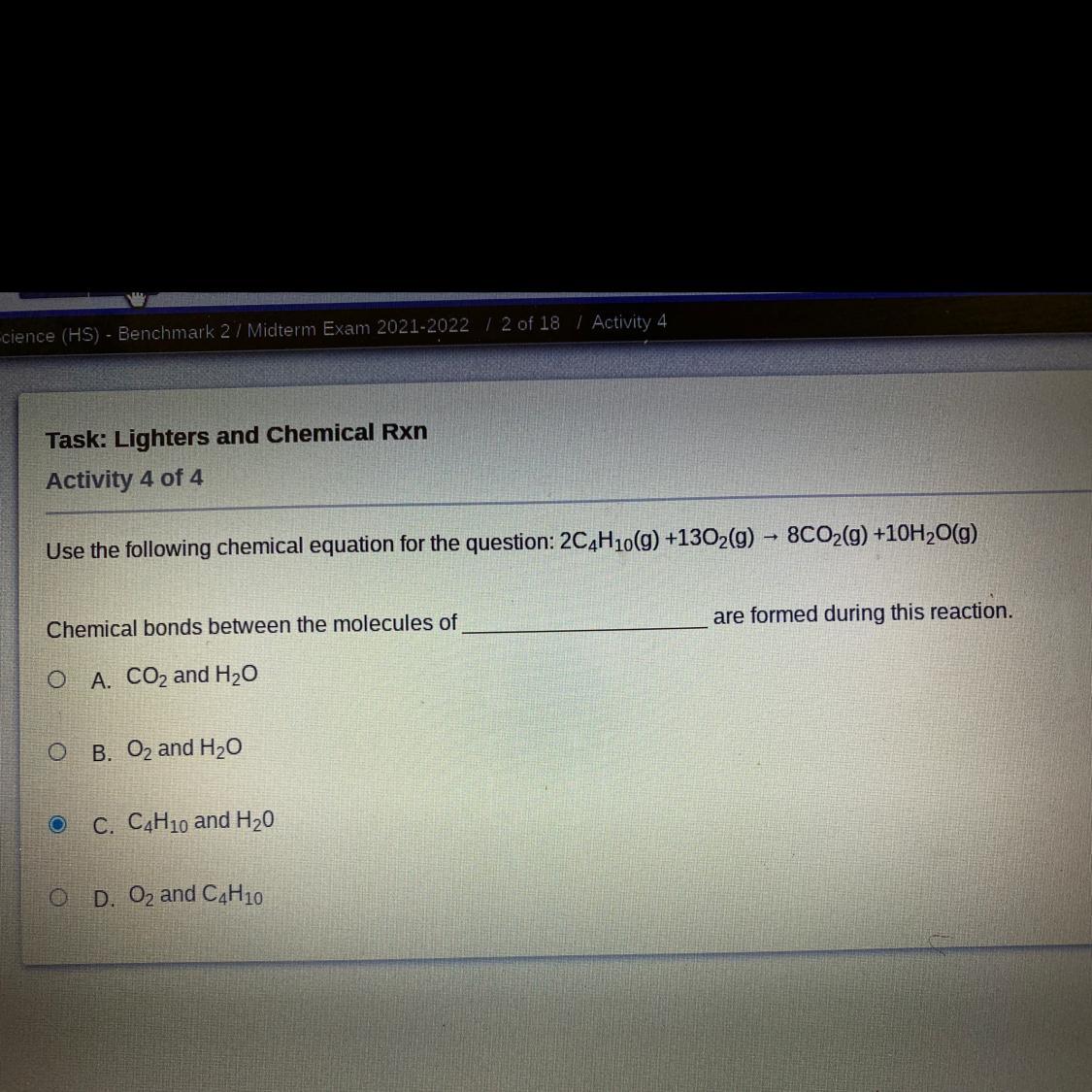
Answers
draw the basic structure of an amino acid. how does the structure of an amino acid contribute to its function? use the terms r group, folding, peptide bond, polarity, ribosome, and carbon.
Answers
The monomers that makeup proteins are amino acids. The basic structure of amino acids is shown below:
One or more polypeptides, which are separate chains of amino acids arranged in a linear way, come together to form a protein. There are 20 different kinds of amino acids that are typically present in proteins.
A core carbon atom, sometimes referred to as the alpha (α) carbon, is joined to an amino group (\(NH_{2}\)), a carboxyl group (COOH), and a hydrogen atom to form the fundamental structure of all amino acids.
The R group, a second atom or group of atoms attached to the central atom in every amino acid, is what gives the amino acid its identity. For instance, the amino acid is glycine if the R group is a hydrogen atom, and alanine if the R group is a methyl \(CH_{3}\) group.
To know more about monomers click on the link below:
https://brainly.com/question/18784783
#SPJ4

Answer asap have to finish by 1

Answers
Answer:
B
Explanation:
because b is way more precise for measuring smaller volumes of liquid
Answer:
B
Explanation:
because b is way more precise for measuring smaller volumes of liquid THANKS 00.0(0 votes)Express your feedback with quick commentsNeed a bit more clarification? Get a high-quality answer with step-by-step explanations from a professional in just minutes instead!GET A VERIFIED ANSWERAdvertisementStill have questions?FIND MORE ANSWERSASK YOUR QUESTIONNew questions in ChemistryWhich water-soluble vitamin cannot be absorbed by the intestinal mucosa unless it has been bound to intrinsic factor?What type(s) of bonds is (are) found between atoms within hydrocarbon molecules?Why are mixtures of gases, such as argon and helium, much more likely to form an ideal solution than other combinations such as solids and liquids?The number of covalent bonds that a nitrogen atom is expected to form is?(CO3) 2 is the _______ group of answer choices acetate cyanide hydrogen carbonate (bicarbonate)carbonateAnswer asap have to finish by 1Divide the scientific notationgood morning another free point question:) have a good life and the person who gave me advice i love youperform the following mathematical operation, and report the answer to the correct numbers of significant figures 2.500 x 10^2 __________= ?x10^? 5….00 x 10^-5What information does an equilibrium constant give about a reaction? A. It tells whether products or reactants are favored at equilibrium. B. It tells…how much energy is required for the reaction to happen. C. It tells how long it takes the reaction to reach equilibrium. D. It tells what the rate constant of the reaction is at equilibrium. SUBMITLOAD MOREPreviousNextplattnicholasrAmbitious2224 pts2Answer MasterNumbers Nerd+16 moreCurrent planBrainly BasicSpecial Offers3View My AchievementsAccount SettingsYour influence2Answering StreakYour Streak is on! Answer another question tomorrow and keep it going.Fun factAn apple, potato, and onion all taste the same if you eat them with your nose plugged 1Fun factBananas are curved because they grow towards the sun ☀️233Get +6 bonus3Last 7 daysOverallYour stats from the last 7 days compared with the previous 7 daysPopularity16People learning from your answers.+1600%Brainliest2This many users said your answers were the best!+200%Thanks7Your effort is appreciated!+700%Questions answered42+4200%17 Aug - 23 Aug
Rank the following gases by the number of moles they would contain at STP, greatest to least.30 L of helium gas, 0.5 moles of oxygen gas, 67.2 L of nitrogen gas.HeliumNitrogen Oxygen
Answers
The ranking from greatest to least number of moles at STP would be: 1. Nitrogen gas (since it has the largest volume of gas at STP), 2. Helium gas (since it has a smaller volume but still more moles than oxygen). 3. Oxygen gas (since it has the smallest number of moles despite its smaller volume)
the gases based on the number of moles at STP. We'll use the Ideal Gas Law (PV=nRT) and the STP conditions (1 atm pressure, 273 K temperature).
1. First, we'll find the number of moles for each gas.
For helium: n = PV/RT
- Given: P = 1 atm, V = 30 L, R = 0.0821 L*atm/(mol*K), T = 273 K
- n(He) = (1 * 30) / (0.0821 * 273) ≈ 1.34 moles
For oxygen: n is already given - 0.5 moles
For nitrogen: n = PV/RT
- Given: P = 1 atm, V = 67.2 L, R = 0.0821 L*atm/(mol*K), T = 273 K
- n(N₂) = (1 * 67.2) / (0.0821 * 273) ≈ 2.99 moles
2. Rank the gases by the number of moles:
- Nitrogen (2.99 moles) > Helium (1.34 moles) > Oxygen (0.5 moles)
So, the ranking from greatest to least number of moles at STP is Nitrogen, Helium, and then Oxygen.
to learn more about Nitrogen click here:
brainly.com/question/14436345
#SPJ11
can anyone show the working steps?

Answers
Volume of potassium hydroxide that can neutralise 25 cm³ of H2Z acid solution is 5cm³
Diprotic acid is that acid which contains two hydrogen atoms and can release or ionise in water, such as carbonic acid and sulphuric acid.A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.The neutralization reaction is
H2Z + 2KOH → K2 H2Z + 2H2O
H2Z acid is diprotic acid as it has two ionizable hydrogens.
Thus, 1 mole of Z acid will neutralize 2 moles of KOH.
The number of moles of phosphorus acid present in 0.025 dm³ of 0.5 M aqueous solution is 0.1×
= 0.0025
They will neutralize 2×0.0025mol = 0.005 moles of KOH.
The molarity of KOH solution is 1.0 mol dm³
The volume of KOH solution required will be
1.0 × 0.005 = 0.005 dm³ = 5cm³
Hence, volume of KOH solution required will be 5 cm³.
learn more about neutralization reaction here:
https://brainly.in/question/15104847
#SPJ9
A population of a wild animal is a frequent target for hunters. Hunters tend to hunt the larger animals with big horns. How might this affect the populations in the wild?
A.
Animals with average horns will increase in number.
B.
Smaller animals with a trait for short horns will become endangered.
C.
Traits for larger animals with big horns will be selected.
D.
Traits for smaller animals with short horns will be selected.
Answers
Traits for larger animals with big horns will be selected.
Which of the diagrams below shows an atom that is not a form of hydrogen?

Answers
Answer:
I believe is the npp i kinda forgot how to do these lol
Diagram with npp type shows an atom that is not a form of hydrogen.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/30898688
#SPJ2
What is silicon on the periodic table?
Answers
Chemical element silicon has the chemical symbol Si and atomic number 14. It belongs to the periodic table's group 14, sometimes known as the carbon group, along with lead, germanium, tin, and carbon. Since silicon
Chemical element silicon has the chemical symbol Si and atomic number It is the second most prevalent element in the Earth's crust, after oxygen and is a hard, brittle crystalline solid with a blue-grey metallic sheen Silicon is a semiconductor, which means that under certain circumstances it can conduct electricity. Silicon is frequently used in electrical devices including transistors, solar cells, and computer processors. Moreover, it's critical to the production of numerous alloysceramics, and glass. In nature, silicon isn't found in its purest form instead, it may be found in a variety of compounds, such silicon dioxide
Learn more about silicon here:
https://brainly.com/question/28213172
#SPJ4
The area of an object is calculated from experimental data to be 24.6623 cm2. The ± absolute error in the area was determined to be ± 0.6 cm2. The area should be reported, in cm 2 , as A. 25 B. 24.7 C. 24.66 D. 24.6623 E. 24.662
Answers
we should take out from point
Which statement best identifies which of these models represents a gas?
Answers
Because its particles move faster as swiftly and are spaced more apart, Diagram B represents a gas.
What is gas ?One of the four basic states of matter, along with solid, liquid, and plasma, is gas. Individual atoms, elemental molecules derived from a single type of atom, or complex molecules derived from a number of atoms can all be found in a pure gas. A variety of pure gases can be found in a gas mixture like air.
By evaluating the four major gases you're searching for—oxygen (O2), carbon monoxide (CO), hydrogen sulphide (H2S), and methane (CH4)—a 4 gas monitor can help you protect your employees in any setting.
Thus, Diagram B is a gas, because its particles move more quickly and are farther apart.
To learn more about gas, follow the link;
https://brainly.com/question/3637358
#SPJ1
Your question is incomplete, probably your question was
Which statement best identifies which of these models represents a gas? Model A is tightly packed, and Model B is spaced out and far apart.
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
how many litres of an 80% acid solution must be added to 4
litres of a 20% acid solution to obtain a 40% solution
Answers
2 liters of the 80% acid solution must be added to 4 liters of the 20% acid solution to obtain a 40% acid solution.
Let's denote the number of liters of the 80% acid solution to be added as x.
To determine the amount of acid in the resulting solution, we can calculate the sum of the acids in the initial solutions and set it equal to the acid in the final solution.
The acid in the 80% solution (0.80x) added to the acid in the 20% solution (0.20 × 4 liters) should be equal to the acid in the final 40% solution (0.40 × (4 + x) liters).
This can be expressed as:
0.80x + 0.20 × 4 = 0.40 × (4 + x)
Simplifying the equation:
0.80x + 0.80 = 1.60 + 0.40x
Combining like terms:
0.80x - 0.40x = 1.60 - 0.80
Simplifying further:
0.40x = 0.80
Dividing both sides by 0.40:
x = 2
Therefore, 2 liters of the 80% acid solution must be added to 4 liters of the 20% acid solution to obtain a 40% acid solution.
To know more about solution :
https://brainly.com/question/29639696
#SPJ4
when sodium chloride reacts with silver nitrate, silver chloride precipitates. what mass of agcl is produced from 143 g of agno3? answer in units of g.
Answers
the mass of AgCl produced from 143 g of AgNO3 can be calculated using stoichiometry.
The balanced chemical equation for the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3) is:
NaCl + AgNO3 → AgCl + NaNO3
From the equation, we can see that one mole of AgNO3 reacts with one mole of NaCl to produce one mole of AgCl. Therefore, we need to use the molar mass of AgNO3 to convert 143 g of AgNO3 to moles, and then use the mole ratio between AgNO3 and AgCl to determine the amount of AgCl produced in moles. Finally, we can convert the moles of AgCl to grams using the molar mass of AgCl.
Step 1: Convert grams of AgNO3 to moles
molar mass of AgNO3 = 107.87 g/mol
moles of AgNO3 = 143 g / 107.87 g/mol = 1.325 mol
Step 2: Use mole ratio to determine moles of AgCl produced
From the balanced equation, the mole ratio between AgNO3 and AgCl is 1:1. Therefore, the amount of AgCl produced in moles is also 1.325 mol.
Step 3: Convert moles of AgCl to grams
molar mass of AgCl = 143.32 g/mol
mass of AgCl produced = 1.325 mol x 143.32 g/mol = 190.0 g
Therefore, the mass of AgCl produced from 143 g of AgNO3 is 190.0 g (in units of grams).
To know more about stoichiometry. , visit
https://brainly.com/question/30215297
#SPJ11