how many pounds of gold are present in 1 cubic mile of seawater?
Answers
Answer:
38 pounds of gold in cubic mile of seawater.
Related Questions
3 hazards that occur in teaching and learning of science
Answers
A silver bracelet was lowered into a graduated cylinder holding a volume of water equal to 10 mL. The height of the water rose to 14 ml . If the mass of the bracket was 42 g,what is the destiny of silver?
Answers
Answer:
2g
Explanation:
Answer:
\(\boxed {\tt 10.5 \ g/mL}\)
Explanation:
Density can be found by dividing the mass by the volume.
\(d=\frac{m}{v}\)
The mass is 42 grams.
Let's find the volume. The volume was found through water displacement, so we must subtract the final volume of the water with the bracelet (14 mL) and the starting volume of water (10 mL)
v= 14 mL-10 mL v= 4 mLNow we know that:
\(m= 42 \ g\\v= 4 \ mL\)
Substitute the values into the formula.
\(d=\frac{42 \ g }{4 \ mL}\)
Divide.
\(d= 10.5 \ g/mL\)
The density of the silver is 10.5 grams per milliliter.
what is the percent yield of carbon disulfide if the reaction of 43.7 g of carbon produces 34.8 g of carbon disulfide? express your answer with the appropriate units. activate to select the appropriates template from the following choices. operate up and down arrow for selection and press enter to choose the input value typeactivate to select the appropriates symbol from the following choices. operate up and down arrow for selection and press enter to choose the input value type percent yield of cs2
Answers
The percent yield of carbon disulfide is 37.5% if the reaction of 43.7 g of carbon produces 34.8 g of carbon disulfide.
To calculate the percent yield of carbon disulfide (CS₂), we need to compare the actual yield of CS₂ obtained from the reaction to the theoretical yield that would be obtained if all of the carbon reacted to form CS₂. The percent yield is then calculated using the following formula;
Percent yield =(actual yield/theoretical yield) x 100%
First, we need to calculate the theoretical yield of CS₂ based on the amount of carbon used in the reaction. Balanced chemical equation for the reaction is;
C + 2S → CS₂
From this equation, we can see that the molar ratio between carbon and CS₂ is 1:1. Therefore, the number of moles of CS₂ that can be produced from 43.7 g of carbon is;
moles of CS₂ = moles of C = mass of C / molar mass of C
= 43.7 g / 12.01 g/mol
= 3.637 mol
Since the molar ratio between carbon and CS₂ is 1:1, the theoretical
yield of CS₂ would be 3.637 mol.
Next, we need to calculate the actual yield of CS₂ obtained from the reaction, which is given as 34.8 g.
Now we can use the formula above to calculate the percent yield;
Percent yield =(actual yield/theoretical yield) x 100%
= (34.8 g / (3.637 mol x 76.14 g/mol)) x 100%
= 37.5%
Therefore, the percent yield of CS₂ is 37.5%.
To know more about percent yield here
https://brainly.com/question/12704041
#SPJ4
help me please. Can you please explain to me how to solve them? you can also send me a photo if that's better for you. thanks alot

Answers
Refer to the attachment

The difference between a conjugate acid/base pair is
+
o the conjugate acid
o the conjugate base
O one proton
one OH-
Answers
Answer:
hola no ablo inglés porfavor
Calculate the solubility of mercury(II) iodide (Hgla) in each situation: a. pure water b. a 3.0 M solution of Nal, assuming (Hg4)2- is the only Hg-containing species present in significant amounts Ksp = 2.9 10-29 for Hgla and K = 6.8 x 1029 for (Hgla)2-.
Answers
The solubility of mercury(II) iodide (HgI₂) in pure water is determined by its Ksp value, which is 2.9 x 10⁻²⁹.
In a 3.0 M solution of NaI, assuming (HgI₄)₂₋is the only significant species, the solubility of HgI₂can be calculated using the Ksp and K values.
What are the solubility values of HgI₂in pure water and a 3.0 M solution of NaI?The solubility of HgI2 in pure water can be calculated using its solubility product constant (Ksp). The Ksp value for HgI₂ is given as 2.9 x 10⁻²⁹. Solubility product constant represents the equilibrium constant for the dissolution of a sparingly soluble salt. By solving the equilibrium expression for HgI₂, we can determine its solubility in pure water.
In a 3.0 M solution of NaI, assuming the formation of (HgI₄)₂₋ is the only significant Hg-containing species, the solubility of HgI2 can be calculated using the Ksp and K values. The K value given for(HgI₄)₂₋ - is 6.8 x 10²⁹. By setting up an equilibrium expression considering the dissociation of HgI₂ into (HgI₄)₂₋ ions, we can determine the solubility of HgI₂in the presence of the NaI solution.
These calculations involve using the principles of equilibrium and the relationship between concentrations of dissolved species and their equilibrium constants. Solubility is defined as the maximum amount of solute that can be dissolved in a given solvent under specific conditions. By applying the relevant equilibrium expressions and values, we can determine the solubility of HgI₂ in each situation.
Learn more about: Solubility
brainly.com/question/31493083
#SPJ11
How do you think that metallic salts are used in fireworks?
Answers
what is the chemical reaction that occurs during photosynthesis
Answers
Answer:
endothermic change occurs
Explanation:
photosynthesis, is an endothermic reaction in which energy is absorbed from the surrounding.
hope this helps
Filter the air that we inhale and exhales carbon dioxide
Answers
Air filters are devices designed to remove impurities and particles from the air, improving its quality and making it healthier to breathe. While air filters can help remove certain contaminants, such as dust, pollen, and pet dander, they do not specifically filter out carbon dioxide (CO2).
Carbon dioxide is a natural component of the air we exhale, and its concentration in the atmosphere is regulated through natural processes, such as photosynthesis by plants. The removal of carbon dioxide from the air typically occurs through the natural carbon cycle, where plants absorb CO2 during photosynthesis and release oxygen.
If you are looking to reduce the carbon dioxide levels indoors, the most effective method is to ensure proper ventilation in the space. This can be achieved by opening windows or using mechanical ventilation systems to bring in fresh outdoor air and remove stale air. Additionally, increasing the number of plants indoors can help absorb carbon dioxide and release oxygen through photosynthesis.
It's important to note that while air filters can improve indoor air quality by removing various pollutants, they are not designed to specifically target or remove carbon dioxide.
Learn more about carbon dioxide Here-
https://brainly.com/question/14445045
#SPJ4
4. You wish to prepare 500. mL of a 0.762 M solution of FeCl3. How many grams of FeCl3 should you weigh out
Answers
To make 500 mL of 0.762 M solution of FeCl₃, the weight of FeCl₃ that should be taken will be 61.91 g.
The molarity of a solution is calculated by the formula:
Molarity = \(\frac{Number of moles of solute}{Volume of solvent}\)
That is, Number of moles of solute = Molarity × Volume of solvent
The given molarity is 0.762 M and the volume to be made is 500 mL which is 0.5 L. Substituting these values in the above equation, we get:
Number of moles of FeCl₃ = 0.762 × 0.5 = 0.381 mol
To find the weight of FeCl₃, we can use the formula for calculating number of moles by rearranging it. That is,
Weight of FeCl₃ = Number of moles × Molecular mass of FeCl₃
Molecular mass of FeCl₃ = 56 + (3×35.5) = 162.5 g/mol
Substituting in above equation,
Weight of FeCl₃ = 0.381 × 162.5 = 61.91 g
Learn more about molarity in:
https://brainly.com/question/31545539
#SPJ4
A blacksmith heated an iron bar to 1445 °C. The blacksmith then tempered the metal by dropping it into 42,800 mL of
water that had a temperature of 22.00 °C. The final temperature of the system was 45.00°C. What was the mass of
the iron bar? (Hint: Density of water is 1.00 g/mL) The specific heat capacity for iron is 25.01 J/mol°C.
Answers
Answer:
6626 g
Explanation:
Given that:
Density of water = 1.00 g/ml, volume of water = 42800 ml.
Since density = mass/ volume
mass of water = volume of water * density of water = 42800 ml * 1 g/ml = 42800 g
Initial temperature of water = 22°C and final temperature of water = 45°C.
specific heat capacity for water = 4.184 J/g°C
ΔT water = 45 - 22 = 23°C
For iron:
mass = m,
specific heat capacity for iron = 0.444 J/g°C
Initial temperature of iron = 1445°C and final temperature of water = 45°C.
ΔT iron = 45 - 1445 = -1400°C
Quantity of heat (Q) to raised the temperature of a body is given as:
Q = mCΔT
The quantity of heat required to raise the temperature of water is equal to the temperature loss by the iron.
Q water (gain) + Q iron (loss) = 0
Q water = - Q iron
42800 g × 4.184 J/g°C × 23°C = -m × 0.444 J/g°C × -1400°C
m = 4118729.6/621.6
m = 6626 g
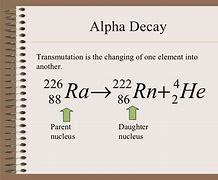
226 • Ra 4/2 He + 88 = ?
Answers
Answer: the answer for this question is in the pic
Explanation:
Density is the ratio of mass to volume. Princess listed the density of four metals at 20 °C.
Material
brass
gold
iron
lead
A. brass
B. gold
C. iron
D. lead
Density (g/cm³)
8.9
about 19.3
7.8
about 11.3
If Princess has a 4-cm 3 cube of each of these metals, which cube will have the greatest
mass?
Answers
The cube of gold will have the greatest mass of 77.2 g.
The correct answer is B.
Which cube will have the greatest mass?The cube that will have the greatest mass is determined from the density of the cubes using the formula below:
Mass = density * volumeFor brass: density = 8.9 g/cm³, volume = 4 cm³
mass = 8.9 g/cm³ x 4 cm³
mass = 35.6 g
For gold: density = about 19.3 g/cm³, volume = 4 cm³
mass = (about 19.3 g/cm³) x 4 cm³
mass = about 77.2 g
For iron: density = 7.8 g/cm³, volume = 4 cm³
mass = 7.8 g/cm³ x 4 cm³
mass = 31.2 g
For lead: density = about 11.3 g/cm³, volume = 4 cm³
mass = (about 11.3 g/cm³) x 4 cm³
mass = about 45.2 g
Learn more about density at: https://brainly.com/question/1354972
#SPJ1
You mix 2 moles of hbr with 3 moles of koh in enough water to make 1 l of solution. how much kbr do you expect to make?
Answers
If we mixed 3 moles of HBr with 2 moles of KOH in enough water to make 1 liter of solution, then the amount of KBr that would be formed would be 3 moles.
The balanced chemical equation for the reaction between HBr and KOH is:
HBr + KOH → KBr + H2O
In the given case, we have 3 moles of HBr and 2 moles of KOH
Their mole ratio = 1.5:1.
This means that for every 1.5 moles of HBr, we have 1 mole of KOH, which will be enough to react with all the HBr.
So, the amount of KBr formed would be 3 moles, which is the same as the amount of HBr that was added to the reaction.
To learn more about potassium hydroxide (KOH):
https://brainly.com/question/28330489
#SPJ4
lonic compounds can conduct electricity in
Answers
Answer:
Ionic compounds conduct electricity when molten (liquid) or in aqueous solution (dissolved in water), because their ions are free to move from place to place. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move
Explanation:
Zinc reacts with Copper (II) chloride to produce Copper and Zinc chloride. -write chemical formula
Answers
Answer:
here's the answer hope it helps

How many grams of sodium are needed to produce 12.5g of sodium oxide
Answers
Answer:
25 possibly
Explanation:
I'm not too sure about this, but sodium oxide is Na2O, 2 sodium and 1 oxygen, so 12.5g * 2 is 25
If someone else comes up with a more convincing argument listen to them
.. When water boils, you can see bubbles rising to the surface of
the water. Of what are these bubbles made?
a. air
b. hydrogen and oxygen gas
c. oxygen gas
d. water vapor
e. carbon dioxide gas
Answers
Answer:
Explanation:
It's water vapor. There is enough heat present to get the water to boil but not enough to break it into its chemical components (oxygen and hydrogen), so the answer is D.
How many grams of rubber stoppers would be needed to contain the same number of stoppers as there are corks in 1.0 kg of corks?
Answers
The weight of each stopper can vary based on the material, size, and manufacturing process used, so it is not possible to determine the number of rubber stoppers needed without more information.
What is weight and average weight?Weight is a measure of the force of gravity on an object and is typically measured in units of Newtons (N), pounds (lb), or kilograms (kg).
Average weight, on the other hand, refers to the sum of the weights of a group of objects divided by the number of objects in the group. It provides an estimate of the typical or central value of the weights in the group. The units of average weight are the same as the units used to measure weight.
The number of rubber stoppers needed to contain the same number of stoppers as there are corks in 1.0 kg of corks would depend on the average weight of each type of stopper.
The weight of each stopper can vary based on the material, size, and manufacturing process used, so it is not possible to determine the number of rubber stoppers needed without more information.
Learn more on average weight here https://brainly.com/question/26952238
#SPJ1
what is the molarity of a solution prepared by dissolving 2.0 g of naoh in water to make a total solution of 250 ml
Answers
The molarity of the solution prepared by dissolving 2.0 g of NaOH in water to make a total solution of 250 mL is 0.2 M.
To calculate the molarity of a solution, we need to know the amount of solute (in moles) and the volume of the solution (in liters).
First, we need to convert the mass of NaOH (2.0 g) to moles. We can do this by dividing the mass by the molar mass of NaOH:
2.0 g NaOH / (40.00 g/mol NaOH) = 0.05 mol NaOH
Next, we need to convert the volume of the solution from milliliters to liters:
250 mL = 0.250 L
Now we can calculate the molarity of the solution:
Molarity = moles of solute / liters of solution
Molarity = 0.05 mol NaOH / 0.250 L solution
Molarity = 0.2 M
To learn more about molarity click on,
https://brainly.com/question/32079676
#SPJ4
How do I write the following :
One atom of Nitrogen
Five atoms of Nitrogen
One molecule of Nitrogen
Five molecules of Nitrogen
One molecule of Sulfur
One atom of Sulfur
Eight molecules of Sulfur
Answers
Answer:
Five molecules of nitrogen and eight molecules of sulfur
Explanation:
Please help me the picture is above.

Answers
Answer:
A
Explanation:
bc inorganic compoud refers to all compound that do not contain carbons.
HELP PLEASE!!! I NEED THE HELP
Which of the following describes the relationship between producers, consumers, and decomposers and how they keep the food web cycle going?
Consumers consume both decomposers and producers. They then die and are replaced by a new generation, starting the cycle again.
Decomposers are broken down and used by producers to produce food for consumers. Consumers are then decomposed, starting the cycle again.
Producers are decomposed by decomposers, which are then consumed by consumers. New producers are born and start the cycle again.
Producers are consumed by consumers only to be decomposed by decomposers. This dead matter is used to feed producers to start the cycle again.
Answers
Producers are consumed by consumers only to be decomposed by decomposers. This dead matter is used to feed producers to start the cycle again.
Answer:
Producers are consumed by consumers only to be decomposed by decomposers. This dead matter is used to feed producers to start the cycle
how many moles of CO2 are in 66.0g of CO2
Answers
Answer:; 66 g is about 3/2 the value of the molar mass of CO2. It is reasonable that the sample contains 3/2 (1.5) mol.
Explanation:
2 NaCl + F2 → 2 NaF + Cl2 The Reactants in this equation are
2 NaCl + F2
2 NaF + Cl2
H2O
NaClF
Answers
The reactants are 2 NaCl + F2
The ____number can vary among atoms of the same element.
Answers
Answer:
Mass number
Explanation:
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons, which would cause a different mass number
Answer:
The answer is mass number
Rates of reaction virtual lab please help I’m so lost!!
Answers
A virtual lab on rates of reaction can be an excellent way to learn about the factors that influence the speed of chemical reactions.
In such a lab, you might be asked to observe the effects of changing variables such as temperature, concentration, and surface area on the rate of a given chemical reaction.
1. Rates of reaction: This refers to the speed at which reactants are converted into products in a chemical reaction. It can be affected by factors such as temperature, concentration, surface area, and catalyst presence.
2. Virtual lab: A virtual lab is an online platform where you can conduct experiments and simulate real-life scenarios in a safe and controlled environment, without the need for physical equipment or materials.
Now, to help you with your virtual lab, you should follow these general steps:
1. Understand the objective: Identify the goal of the experiment, such as determining the effect of a specific factor on reaction rates
2. Set up the experiment: Select the appropriate reactants, concentrations, and other conditions needed to perform the experiment in the virtual lab enzymes.
3. Conduct the experiment: Observe the virtual lab's interface to see how the reaction progresses and collect data, such as reaction time or product formation.
4. Analyze the results: Compare the collected data to identify trends or patterns, and draw conclusions about how the factors investigated impact the reaction rate.
5. Repeat as needed: Perform additional experiments to test different conditions and validate your findings.
Learn more about enzymes here
https://brainly.com/question/28496090
#SPJ11
The complete question is
Describe rates of reaction in virtual lab?
Water's unusual properties are primarily due to
a molecular geometry
b small molar mass
c chemical reactivity
d hydrogen bonding
Answers
Answer:
D hydrogen bonding
Explanation:
A gas with a pressure of 820.4 mmHg occupies a
volume of 900.0 mL at a temperature of 25.0°C. If
the pressure does not change, what is the new
volume of the gas at 132.0°C?
A) 1220 L
B) 4750 L
C) 4750 mL
D) 1220 mL
Answers
Answer:
V₂ = 1223.2 mL
Explanation:
Given data:
Pressure of gas = 820.4 mmHg
Initial volume of gas = 900.0 mL
Initial temperature = 25.0°C (25+273=298K)
Final temperature = 132.0°C (132.0 +273 = 405 K)
Final volume = ?
Solution:
Solution:
The given problem will be solve through the Charles Law.
According to this law, The volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure.
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 900.0 mL × 405 K / 298 k
V₂ = 364500 mL.K / 298 K
V₂ = 1223.2 mL
Answer fast! All transition metals have _______ valence electrons.
Answers
Answer:
2 Answers. Most transition metals have 2 valence electrons. Valence electrons are the sum total of all the electrons in the highest energy level (principal quantum number n). Most transition metals have an electron configuration that is ns2(n−1)d , so those ns2 electrons are the valence electrons.
Explanation: