Answers
Answer:
8 protons, 10 electrons.
Explanation:
the -2 charge adds 2 electrons–hence, you have 10 electrons while protons remain at 8.
Related Questions
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
What is the kinetic energy of a 328 kg object that is moving at s speed of 10 m/s
Answers
16400 Joules is the kinetic energy of a 328kg object with a velocity of 10m/s.
What is the kinetic energy of the object?Kinetic energy is simply energy possessed by a body in motion.
Kinetic energy is expressed as;
Kinetic energy = 1/2 × m × v²
Where v is velocity and m is mass of the object,
Given the data in the question;
Mass of the object m = 328kgVelocity of the object = 10m/sKinetic energy = ?To determine the kinetic energy of the object, plug the given values into the formula above.
Kinetic energy = 1/2 × m × v²
Kinetic energy = 1/2 × 328kg × (10m/s)²
Kinetic energy = 1/2 × 328kg × 100m²/s²
Kinetic energy = 328kg × 50m²/s²
Kinetic energy = 16400kgm²/s²
Kinetic energy = 16400J
Therefore, the kinetic energy 16400 Joules.
Learn more about kinetic energy here: https://brainly.com/question/27397088
#SPJ1
Draw a second resonance structure for the following ion (be sure to include the charges and all lone pairs)... + ..N=N=N <-------------->.. ..
Answers
Answer:
Explanation:
Resonance structure occurs in an organic compound that undergoes resonance effects. This resonance effect is sometimes called the mesomeric effect helps to increases the stability of organic compounds that have alternating single bonds and double bonds.
The second resonance structure diagram for the ion given in the question can be found in the attached diagram below.

Consider the precipitation reaction: BaCl 2 + 2 AgNO 3 → 2 AgCl + Ba(NO 3) 2. How many grams of AgCl are generated when 85 g of BaCl 2 reacts?
Answers
The grams of AgCl are generated when 85 g of BaCl₂ reacts is 10.86 g.
The balanced equation is given as :
BaCl₂ + 2AgNO₃ -----> 2AgCl + Ba(NO₃)₂
the mass of the BaCl₂ = 85 g
molar mass of BaCl₂ = 208.23 g/mol
moles of BaCl₂ = mass / molar mass
= 85 / 208.23
= 0.408 mol
1 mole of BaCl₂ produce 2 moles of AgCl
0.408 moles of BaCl₂ = 2 × 0.408
= 0.816 mol of AgCl
moles of AgCl = 0.816 mol
mass of AgCl = moles × molar mass
= 0.816 g × 143.32 g/mol
= 10.86 g
Thus, The grams of AgCl are generated when 85 g of BaCl₂ reacts is 10.86 g.
To learn more about moles here
https://brainly.com/question/26416088
#SPJ9
I will mark brainliest as soon as there are two answers! Please be quick, I'm timed
A mineral forms in water heated by magma inside Earth.
Which statement best describes this mineral?
The mineral formed through evaporation.
The mineral crystallized from molten material.
The mineral formed when a hot water solution cooled.
The mineral likely has all the same properties as the surrounding rock.
Answers
Answer: i would do the mineral formed when a hot water solution cooled because you said that a mineral forms in water heated by magma inside Earth.
Explanation:
The answer would be C
Explanation:
When magma cools down it will change into a rock type so the answer would be C.
What is the rate at which Br⁻(aq) disappears in the reaction below if the rate of disappearance of BrO₃⁻(aq) is 0.041 M/s?
BrO₃⁻ + 5 Br⁻ + 6 H⁺ → 3 Br₂ + 3H₂O
Answers
The rate of disappearance of Br- is 5 times of the rate of disappearance of BrO₃⁻. Hence, the disappearance rate of Br- in the reaction is 0.201 M/s .
What is rate of a reaction ?The rate of a reaction is the rate of disappearance of the reactants or the rate of appearance of the products. The rate of a reaction is directly proportional to the molar concentration of the reactants.
For the given reaction, the rate of reaction can be written in the following terms.
-d/dt [BrO₃⁻] = -1/5 d/dt [Br-] = -1/6d/dt [H+ ] = +1/3d/dt [Br²] = + 1/3 d/dt[H₂O]
The minus sign indicates the disappearance and + indicates the formation.
Given, the rate of disappearance of BrO₃⁻ = -d/dt [BrO₃⁻] = 0.041 M/s.
-d/dt [BrO₃⁻] = 1/5 d/dt [Br-]
then d/dt [Br-] = 5 -d/dt [BrO₃⁻] = 5 × 0.041 M/s = 0.201 M/s.
Therefore, the rate of disappearance of Br- in this reaction is 0.201 M/s.
Find more on reaction rate :
https://brainly.com/question/29261432
#SPJ9
1 points
A bottle contains a mixture of two gases: Oxygen and Hellum. The partial pressure of O2 is 1.0 atm and the partial pressure of He is 100.0 mmHg. What is the total pressure in the tank? (Volume and temperature are
constant)
101 alm
011 atm
O 101 mmHg
O 1.1 mmHg
Answers
101 mmHg is the total pressure in the tank.
Thus, Dalton's Law of Partial Pressures states that while the volume and temperature of a gaseous mixture are held constant, the total pressure of the mixture is equal to the sum of the partial pressures of its gaseous components.
Nitrogen, oxygen, argon, carbon dioxide, water vapor, and a trace amount of other gases are all present in atmospheric air and pressure.
The increased oxygen content in the chamber can displace the CO bound to hemoglobin faster than air oxygen, hence the hyperbaric chambers are also used to treat carbon monoxide (CO) poisoning. The treatment of scuba divers with the bends is another application for hyperbaric chambers.
Thus, 101 mmHg is the total pressure in the tank.
Learn more about Pressure, refer to the link:
https://brainly.com/question/30673967
#SPJ1
Assume that 0.491 g of diborane is combusted in a calorimeter whose heat capacity (Ccalorimeter) is 7.854 kJ/°C at 19.63°C. What is the final temperature of the calorimeter?
Answers
Answer:
The combustion of diborane (B2H6) is as follows:
2B2H6(g) + 6O2(g) → 4H2O(g) + B4O(g)
The balanced chemical equation shows that 2 moles of B2H6 react with 6 moles of O2 to produce 4 moles of H2O and 1 mole of B4O. We can use this information to calculate the amount of heat released by the combustion of 0.491 g of B2H6:
0.491 g B2H6 × (1 mol B2H6/27.67 g B2H6) × (1 mole B4O/2 moles B2H6) × (-2037 kJ/mol B4O) = -7.89 kJ
The negative sign indicates that the reaction releases heat.
The heat released by the reaction is absorbed by the calorimeter, which causes its temperature to increase. We can use the equation:
q = Ccalorimeter × ΔT
where q is the amount of heat absorbed by the calorimeter, Ccalorimeter is the heat capacity of the calorimeter, and ΔT is the change in temperature of the calorimeter.
Rearranging the equation, we get:
ΔT = q/Ccalorimeter
Substituting the values we obtained, we get:
ΔT = (-7.89 kJ)/(7.854 kJ/°C) = -1.005°C
The negative sign indicates that the temperature of the calorimeter decreases by 1.005°C. Therefore, the final temperature of the calorimeter is:
19.63°C - 1.005°C = 18.625°C
Rounding to the appropriate number of significant figures, the final temperature of the calorimeter is 18.6°C.
Hope this is what you are looking for.
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
write the formula the alkenes that contain four,six,eight and ten carbon atoms
Answers
So, if it has 4 carbon atoms it would be C4H8
Six carbon atoms - C6H12
Eight - C8H16
Ten - C10H20
Answer:
??????????????????????????
match the number of negative charges to the number of positive charges to make each combination balanced (see image for answer) just say like thanks, or a fun fact or something for ten points in the answers lol

Answers
Answer : The correct match is:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Explanation :
As we now that there are three subatomic particles which are protons, electrons and neutrons.
The protons and neutrons are located inside the nucleus and electrons are located around the nucleus.
The protons are positively charged, the electrons are negatively charged and neutrons are neutral.
As we know that all the things are made up of charges and opposite charges attract to each other.
In a neutral atom, the positive charges and negative charges are balanced in an object. That means, in neutral atom the number of positive charges are equal to the negative charges.
So we can say that:
1 positive charge = 1 negative charge
2 positive charges = 2 negative charges
3 positive charges = 3 negative charges
Answer:
1 positive---- 1 negative
2 positive---- 2 negative
3 positive---- 3 negative
Explanation:
edge 2023
Which are examples of a phase change? (Select all that apply.)
evaporating water
cutting wood
frying eggs
melting butter
Answers
Answer:
melting butter and frying eggs
Explanation:
Hope this helps please mark me brainliest
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
2NO(g) + Cl₂(g) →→→ 2NOCI(g)Experiment230.0125M0.0125M0.0250MInitial [NO]a) Write the rate law equation for the reaction.b) What is the overall order of the reaction?0.0255M0.0510N0.0255N
![2NO(g) + Cl(g) 2NOCI(g)Experiment230.0125M0.0125M0.0250MInitial [NO]a) Write the rate law equation for](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/LF5Sa4hFou7ywNGAQpllv9Sx7FBkUts3.jpeg)
Answers
Answer:
\(\begin{gathered} a)\text{ Rate = k }\times\text{ \lbrack NO\rbrack}^2\text{ }\times\text{ \lbrack Cl}_2] \\ b)\text{ Order = 3} \end{gathered}\)Explanation:
Here, we want to deduce the rate law for the reaction given
According to the data provided:
\(Rate\text{ = k\lbrack NO\rbrack}^a[Cl_2]\placeholder{⬚}^b\)where the values in the square parentheses represent the concentrations and k represents the rate constant
Let us work with equations 1 and 3:
\(\begin{gathered} 2.27\text{ }\times10^{-5}\text{ = k }\times\text{ 0.0125}^a\text{ }\times\text{ 0.0255}^b \\ 9.08\text{ }\times\text{ 10}^{-5}\text{ = k }\times\text{ 0.0250}^a\text{ }\times\text{ 0.0255}^b \end{gathered}\)Divide equation 3 by 1:
\(\begin{gathered} 4\text{ = 2}^a \\ a\text{ = 2} \end{gathered}\)To get b, we can use equations 1 and 2:
\(\begin{gathered} 2.27\text{ }\times\text{ 10}^{-5}\text{ = k }\times\text{ 0.0125}^a\text{ }\times\text{ 0.0255}^b \\ 4.55\text{ }\times\text{ 10}^{-5}\text{ = k }\times\text{ 0.0125}^a\text{ }\times\text{ 0.0510}^b \end{gathered}\)Divide equation 2 by 1, we have it that:
\(\begin{gathered} 2\text{ = 2}^b \\ b\text{ = 1} \end{gathered}\)The rate law for the reaction is thus:
\(Rate\text{ = k }\times\text{ \lbrack NO\rbrack}^2\text{ }\times\text{ \lbrack Cl}_2]\)b) The overall order is the sum of the powers
That would be 1 + 2 = 3
2 FeCl3 + 3 Pb(NO3)3 -> 2 Fe(NO3)3 + 3 PbCl₂
How many mol of Fe(NO3)3 will be produced by using 1.5 mol of Pb(NO3)3?
Answers
2 FeCl3 + 3 Pb(NO3)2 → 2 Fe(NO3)3 + 3 PbCl2
This equation shows that 2 moles of Fe(NO3)3 are produced for every 3 moles of Pb(NO3)2 consumed.
So, if we start with 1.5 moles of Pb(NO3)3, we can calculate the number of moles of Fe(NO3)3 produced as follows:
(1.5 mol Pb(NO3)3) x (2 mol Fe(NO3)3 / 3 mol Pb(NO3)3) = 1.0 mol Fe(NO3)3
Therefore, 1.0 mole of Fe(NO3)3 will be produced by using 1.5 moles of Pb(NO3)3.
Please help asap!! Need help for problem #2.
Naturally occuring iron, contains 5.82% ^54Fe, 91.66% ^56Fe, 2.19% ^57Fe, and 0.33% ^58Fe. The reslective atomic masses are 53.940 amu, 55.935 amu, 56.935 amu and 57.933 amu. Calculate the average atmoic mass of iron. (show work)

Answers
Answer:
55.56 amu
Explanation:
Let A, B, C and D represent the four isotopes of iron.
The following data were obtained from the question:
Isotope A (Fe-54):
Mass of A = 53.940 amu
Abundance (A%) = 5.82%
Isotope B (Fe-56):
Mass of B = 55.935 amu
Abundance (B%) = 91.66%
Isotope C (Fe-57):
Mass of C = 56.935 amu
Abundance (C%) = 2.19%
Isotope D (Fe-58):
Mass of D = 57.933 amu
Abundance (D%) = 0.33%
Average atomic mass =.?
The average atomic mass of the iron can obtained as follow:
Average atomic mass = [(mass of A × A%)/100] + [(mass of B × B%)/100] + [(mass of C × C%)/100] + [(mass of D × D%)/100]
Average atomic mass = [(53.940 × 5.82)/100] + [(55.935 × 91.66 )/100] + [(56.935 × 2.19)/100] + [(57.933 × 0.33)/100]
= 2.848 + 51.270 + 1.247 + 0.191
= 55.56 amu
Therefore, the average atomic mass of the iron is 55.56 amu
Why does heating increase the speed at which a solute dissolves in water?
O A. It decreases the surface area of the solute.
B. It prevents the solute from cristallizing.
O c. It makes the water molecules move faster.
D. It raises the pressure of the water molecules.
Answers
Answer: C
Explanation:
It gives kinetic energy to the molecules and it breaks the bonds faster because they jiggle more
HELP! I DON'T UNDERSTAND!?

Answers
Answer:
DECREASES THIS IS OBVIOUS
Explanation:
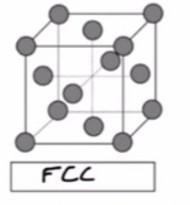
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure). How many Cl- ions are present in one unit cell of this crystal?
Answers
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure). The numbers of Cl- ions present in one unit cell of this crystal is 8.
The Cubic system arrangement is one in which the unit cell is shaped like a cube.
A face centered cubic arrangements (FCC) is an atom arrangement located in nature. FCC structural unit cell is made up of atoms organized in a cube with fraction of an atom in each corner and six additional whole atoms in the middle of the cube face.
From the information given;
Ba2+ is found in the FCC arrangementsThe total number of Ba2+ atom present within the unit cell can be computed as:
\( \mathbf{(6 \ face \: \: sharing \: atom)( \dfrac{1}{2}) \: + (8 \: edges)( \dfrac{1}{8} \: corner \: sharing \: atoms)}\)
\( \mathbf{ = 4 \: atoms}\)
From the formula for BaCl2; since there is twice number of Cl- present as that of Ba2+;
Then, the number of Cl- present per unit cell is:= 4 × 2= 8Therefore, we can conclude that the numbers of Cl- ions present in one unit cell of this crystal is 8.
Learn more about cubic arrangements here:
https://brainly.com/question/21826251?referrer=searchResults
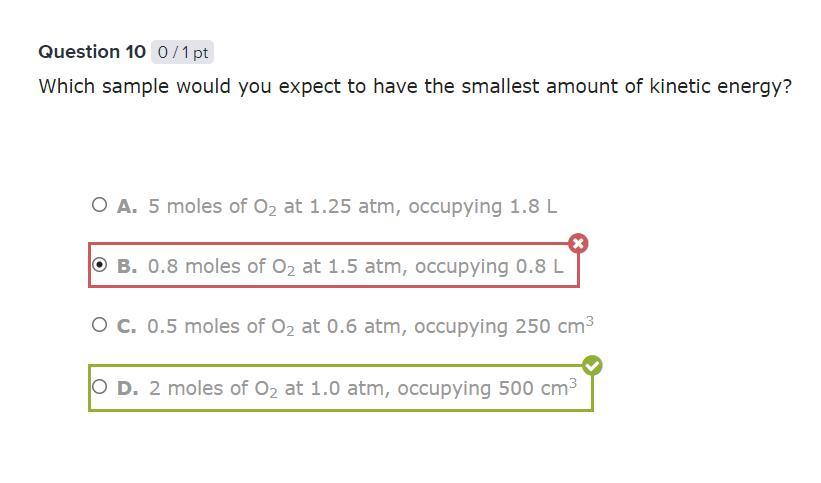
Which sample would you expect to have the smallest amount of kinetic energy?
THIS IS THE ANSWER. NOT A QUESTION
Answers
Answer:
ok so what do i do now that you posted the answer?
Answer: D
Explanation:
definitely didn't copy you
The scientific theory of plate tectonics states that the Earth's crust is composed of many plates, or slabs of rock. Interactions of these plates have created different landforms, or geologic features. Which of the following statements provides evidence for this theory?
A.
A large earthquake off the coast of Japan induces a wave that is over twenty feet high.
B.
An iceberg buckles the hull of the Titanic and allows water to enter between the steel plates.
C.
The city of Pompeii is perfectly preserved under volcanic ash following the eruption of Mount Vesuvius.
D.
Fossilized marine creatures can be found at the top of the Himalayan Mountains.
Answers
Answer:
study island
proof
Explanation:

Fossilized marine creatures found at the top of the Himalayan Mountains provide evidence for a scientific theory of plate tectonics. Therefore, option (D) is correct.
What is Plate tectonics?Plate tectonics can be explained as the Earth's lithosphere being made of a large number of tectonic plates that have been slowly moving since about 3.4 billion years ago.
The lithosphere is broken into 7 or 8 major plates and many minor plates or "platelets". These plates meet, and their relative motion evaluates the kind of plate boundary: convergent, transform, or divergent.
Mountain-building, Earthquakes, volcanic activity, and oceanic trench formation take place along these plate boundaries. The relative annual movement of the plates ranges from 0 to 10 cm.
Tectonic plates are comprised of the oceanic and the thicker continental lithosphere. The process of subduction which is actually one plate moving under another carries the edge of the lower plate down into the mantle along convergent boundaries.
Pebbles, Shells, and marine fossils found in the limestone beds of the tallest Himalayas Mountains give evidence of the theory of plate tectonics.
Learn more about plate tectonics, here:
brainly.com/question/19317822
#SPJ6
what are chemistry products
Answers
Answer:
Products are the species formed from chemical reactions. During a chemical reaction reactants are transformed into products after passing through a high energy transition state. This process results in the consumption of the reactants.
Explanation:
hope this helps if not let me know have a great day
Our 4 seasons are caused by
Answers
Answer: Earth and the sun
Explanation:
Because the earth is tilt toward the sun the planet rotates about axis but it isn't visible,also at different times of the year the northern ans southern axis are close to the sun.
hope this helps
Please help me on this

Answers
The light colored panel reflects light and heats up, while the dark colored panel absorbs the light and stays cooler. This temperature difference causes the vanes to spin.
Describe how the light and dark colored panels affect how the radiometer spins.The dark panel absorbs the incoming light, while the light panel reflects it. Due to this, the two panels' temperatures differ, with the dark panel becoming hotter than the light panel.
Due to the pressure difference caused by the temperature difference, air molecules move from the hot, dark panel to the cool, light panel. The radiometer's associated vanes spin as a result of this air flow.
The radiometer will spin more quickly the greater the temperature differential between the two panels. The temperature differential between the two panels is higher in a vacuum, enabling the radiometer to spin more quickly.
To learn more about temperature difference refer :
https://brainly.com/question/23285916
#SPJ1
Put the steps of the carbon cycle in order using Step 1 as your starting point.
Step 1: Bacteria, through nitrogen fixation and nitrification, convert nitrogen into a usable form.
The animal dies and decomposes, returning nitrogen back to the soil.
Once nitrogen is in usable form, it is taken up by plants and assimilated into proteins..
An animal eats a plant and the nitrogen becomes part of the animal’s proteins.
Answers
Answer:
Carbon moves from the atmosphere to plants. ...
Carbon moves from plants to animals. ...
Carbon moves from plants and animals to soils. ...
Carbon moves from living things to the atmosphere. ...
Carbon moves from fossil fuels to the atmosphere when fuels are burned. ...
Carbon moves from the atmosphere to the oceans.
Explanation:
Answer:
step 4 , 2 , 3
Explanation:
How does size affect weight?
(20 points, write at least 5 sentences answer pls thx!)
Answers
Citric acid is made by oranges. When citric acid dissolved in water , it produces a relatively small number of hydronium ions. Citric is best described as a
Answers
Answer:
Citric acid is a weak organic acid found in citrus fruits.
Explanation:
what is Quantum mechanics??
Can some one summarize this?
Answers
Answer:
its a theory
Explanation:
dealing with the behaviour of matter and light on the atomic and subatomic scale.
Predict the product of each monosaccharide oxidation reaction.

Answers
The complete oxidation of the monosaccharide shown will create a carboxylic acid.
Monosaccharides can be oxidized to carboxylic acids. Recall that monosaccharides have an aldehyde or ketone group at one end and a CH2OH group at the other end.
For the monosaccharide shown, oxidation may lead to the conversion of COH group to acid (-COOH). This confirms the presence of -COH yielding the product C4H8O5.
Learn more: https://brainly.com/question/2192784
A volume of 56.0 mL of He at STP. a. How much weight?
Answers
We already know that number of moles is 0.0025 mol.
So if we know the volume of the gas at STP and the number of moles we can get the mass of the gas.
V = 0.056 L
1 mol = 22.4 L
Molar mass of He = 4.0026 g/mol
So to calculate the weight we need number of moles and molar mass.
weight = 0.0025 mol x 4.0026 g/mol
= 0.01 g