How much heat is absorbed when 70 grams of
water is completely vaporized at its boiling
point?
Answers
Answer:
Thus, the joules of heat absorbed when 70.0 grams of water is completely vaporised at its boiling point are 158,200.
Related Questions
The mass of a 42he nucleus is _____ the sum of the masses of two protons and two neutrons, all separated from each other.
Answers
The mass of a 42He nucleus is slightly less than the sum of the masses of two protons and two neutrons, all separated from each other.
The mass of a 42He nucleus, also known as an alpha particle, is slightly less than the combined mass of two protons and two neutrons. This can be attributed to the phenomenon called mass defect. In an atomic nucleus, the protons and neutrons are held together by the strong nuclear force, which overcomes the electromagnetic repulsion between the positively charged protons. However, this binding force comes at the expense of a small amount of mass.
According to Einstein's mass-energy equivalence principle (E=mc²), mass can be converted into energy and vice versa. The strong nuclear force involved in binding protons and neutrons within a nucleus results in a small decrease in mass. This mass defect is then converted into binding energy, which keeps the nucleus stable.
When two protons and two neutrons come together to form a 42He nucleus, a small amount of mass is lost, corresponding to the energy released as binding energy. This decrease in mass is due to the conversion of mass into energy, as described by Einstein's equation. Therefore, the mass of the 42He nucleus is slightly less than the sum of the masses of its constituent particles when they are separated from each other.
Learn more about the Mass defect
brainly.com/question/4163502
#SPJ11
The electron configuration of nitrogen (N) is 1s22s22p3 1s22s22p4 1s22s22p5 1s22s22p6
Answers
Answer:
option A
\( {1s}^{2} {2s}^{2} {2p}^{3} \)
Explanation:
Atomic number of Nitrogen (N) = 7
So, no. of protons and electrons = 7
Then,
\(1s {}^{2} {2s}^{2} {2p}^{3} \)
Answer:
A: on EDGE :)
Explanation:
Which statement about the electron-cloud model is true?
It is the currently accepted atomic model.
It can easily be replaced by existing models.
It specifies the location and momentum of an electron.
It does not explain the formation of emission lines.
DONT WATCH AN AD ITS (A)!
Answers
Explanation:
The statement "It is the currently accepted atomic model" is true. The electron-cloud model, also known as the electron cloud or electron orbital model, is the currently accepted model of the atom. It describes the behavior of electrons in an atom by representing them as existing in regions of high probability called electron clouds or orbitals. This model successfully explains many properties and behaviors of atoms and has been widely accepted by the scientific community.
A sample of nitrogen gas inside a sealed container with a volume of 6.0 liters and temperature of 100 K exerts a pressure
of 1.50 atm. What pressure will be exerted by the gas if the volume is decreased to 2.0 liters and the temperature
decreases to 75 K?
A. 3.4 atm
B. 0.22 atm
C. 1.5 atm
D. 3.0 atm
Answers
Answer:
The relationship between pressure, volume, and temperature can be described by the ideal gas law:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature.
Assuming that the number of moles, n, and the gas constant, R, remain constant, we can use the combined gas law to solve for the final pressure:
(P1V1)/T1 = (P2V2)/T2
Plugging in the given values, we get:
(1.50 atm x 6.0 L)/100 K = (P2 x 2.0 L)/75 K
Solving for P2, we get:
P2 = (1.50 atm x 6.0 L x 75 K)/(2.0 L x 100 K) ≈ 3.4 atm
Therefore, the answer is A. 3.4 atm.
"You have a solution of glucose in water that has a concentration of 2.50 M and a volume of 0.442 liters. You dilute this solution with water to make a total volume of 1.50 liters. What is the final concentration of this solution?"
Answers
Answer:
0.737M
Explanation:
C1V1=C2V2
2.50 x 0.442 = C x 1.5
1.105 = C x 1.5
C = 1.105/1.5
C = 0.737M
Do the middle one, will give brainliest

Answers
Answer:
it answer is (B)
the formula of toluene is C6H5CH3
it consists of a benzene ring with methyl group attached to it

What is this help ASAP
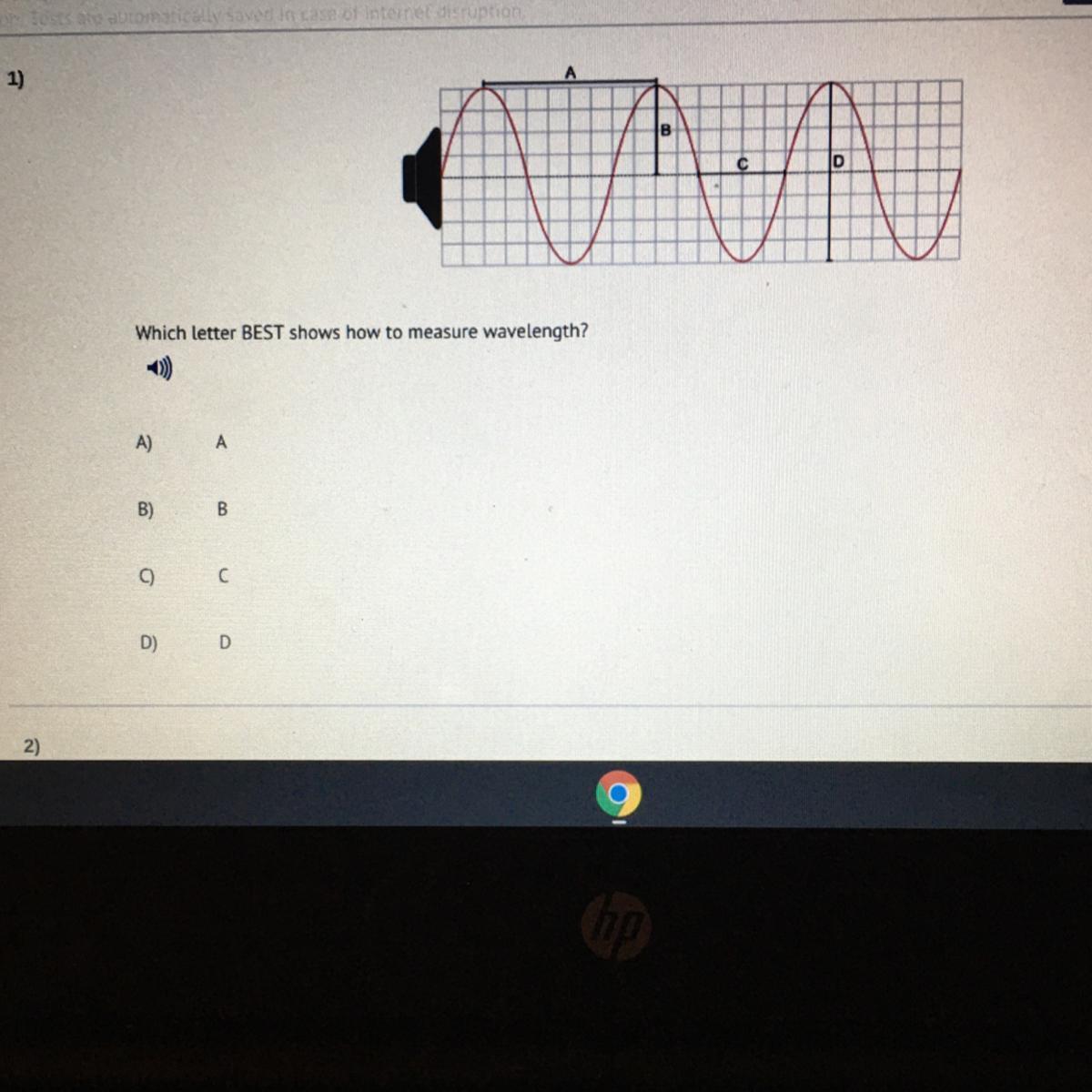
Answers
Answer:
A, im gonna say. Hopefully this helps!
Explanation:
Length is horizontal, and A is horizontal too.
How many moles of butane do we have if we have 5.50 x 10^24 molecules of butane (C4H10)?
Answers
9.14moles
Explanations:According to the Avogadro's constant,
\(1mole\text{ of }C_4H_{10}=6.02\times10^{23}molecules\)We are to determine the moles of 5.50 x 10^24 molecules of butane. This is expressed as:
\(\begin{gathered} mole\text{ of }C_4H_{10}=\frac{5.50\times10^{24}}{6.02\times10^{23}} \\ mole\text{ of C}_4H_{10}=0.914\times10^{24-23} \\ mole\text{ of C}_4H_{10}=0.914\times10 \\ mole\text{ of C}_4H_{10}=9.14moles \end{gathered}\)Therefore the required mole of butane is 9.14moles
In order to have a more observable reaction, a student decides to add some deionized water to the Erlenmeyer flask. How will the experimental [HCl] be skewed
Answers
Adding deionized water to the Erlenmeyer flask will dilute the concentration of HCl in the solution. When deionized water is added to the flask, it increases the volume of the solution without changing the amount of HCl present. As a result, the concentration of HCl will decrease.
The concentration of a solution is defined as the amount of solute (in this case, HCl) dissolved in a given volume of solvent (in this case, deionized water). By adding more deionized water, the total volume of the solution increases, but the amount of HCl remains the same. Therefore, the concentration of HCl will decrease.
The effect of dilution on the concentration of a solution is described by the equation C1V1 = C2V2, where C1 and V1 represent the initial concentration and volume, and C2 and V2 represent the final concentration and volume. In this case, the initial concentration of HCl will be higher before adding deionized water, and the final concentration will be lower after adding deionized water.
In conclusion, adding deionized water to the Erlenmeyer flask will decrease the concentration of HCl, resulting in a skewed experimental [HCl].
To know more about HCl refer to:
https://brainly.com/question/27576431
#SPJ11
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
Mass connects which two things?
Answers
Mass connects: Matter and Energy.
Explanation:
Hope this answer helps.
Mass connects matter and energy.
What does mass connect?Mass relates matter with energy.
In the law of conservation of mass, matter and energy can also effectively function.
Mass can neither be created nor destroyed in reactions. Neither is matter and energy.
Thus, the three quantities are related in a 3-dimensional way. Mass can be converted to matter and energy and vice versa.
More on mass can be found here: https://brainly.com/question/19694949
#SPJ2
What expressions give shte amonunt of light energy that is conerted to oteer forms between the fluresnce excitation anf emission events?
Answers
The expressions which give something amount of light energy that is converted to other forms between the fluorescence excitation and emission events is:
E = hf = hc/λ
Where;
h = 6.62 × 10 −34 Js
c = 3 × 10 8 m/s.
What is energy?Energy can simply be defined as the capacity of doing work.
There are different forms or types of energy. These are:
Mechanical energyLight energyNuclear energyKinetic energyPotential energySo therefore, the expressions which give something amount of light energy that is converted to other forms between the fluorescence excitation and emission events is:
E = hf = hc/λ, where h = 6.62 × 10 −34 J and c = 3 × 10 8 m/s.
Learn more about energy:
https://brainly.com/question/13439286
#SPJ1
what property of does honey possess that makes it resist from flowing
Answers
in form of solid form honey possess
Answer:
Honey
Explanation:
Honey is an ancient remedy for the treatment of infected wounds, which has ... The antimicrobial properties of honey have been known to humans for ...
Abstract
Introduction
Composition
Honey production and ...
Physical properties of honey
Honey as food
Microbes in honey
What step in the water treatment process involves the removal of sediment?
A.
aeration
B.
desalination
C.
filtration
Answers
Which statement accurately describes a type of potential energy found in a container full of a chemical substance in liquid form?
The rotation of the particles is one place where potential energy is stored.
The vibration of the atoms in molecules is one place where potential energy is stored.
The speed of the particles is one place where potential energy is stored.
The bonds between atoms are one place where potential energy is stored.
Answers
Among the options provided, the statement that accurately describes a type of potential energy found in a container full of a chemical substance in liquid form is: "The bonds between atoms are one place where potential energy is stored."
In a container filled with a chemical substance in liquid form, the potential energy is primarily stored in the bonds between atoms within the molecules of the substance. These bonds are formed by the sharing or transferring of electrons between atoms, creating a stable arrangement. The potential energy arises from the electrostatic interactions between the charged particles involved in these chemical bonds.When the liquid substance is in its equilibrium state, the potential energy stored in the bonds represents the energy required to break these bonds and separate the atoms, transforming the substance into a different state (such as a gas or a solid).
This potential energy is released when the substance undergoes a chemical reaction or when external forces act upon it.While other forms of potential energy can also exist in a liquid substance, such as kinetic energy due to the speed of the particles or vibrational energy of the atoms within the molecules, these are generally associated with other types of energy rather than potential energy.
for such more questions on energy
https://brainly.com/question/19666326
#SPJ8
Which of the following statements does NOT support the Kinetic Molecular Theory?
Answer
A
the particles of an ideal gas are separated by short distances, compared to their size.
B
the particles of an ideal gas move in a completely random way.
C
the particles of an ideal gas have no intermolecular forces.
D
the particles of an ideal gas have kinetic energy
Answers
The statements that does not support the Kinetic Molecular Theory is (option A) the particles of an ideal gas are separated by short distances, compared to their size.
What is the Kinetic Molecular Theory?The kinetic molecular theory is a scientific model that explains the behavior of gases based on the motion of their constituent particles. It makes several assumptions about the behavior of gas molecules and how they interact with each other and their surroundings.
In an ideal gas, the particles are considered to be point masses that have negligible volume and do not interact with each other except through elastic collisions.
Therefore, the particles of an ideal gas are considered to be separated by long distances, because there is no attraction or repulsion between the particles, and they move freely in a random motion in the available volume of the container.
Learn about kinetic molecular theory here https://brainly.com/question/134712
#SPJ1
Câu 1. Công thức hoá học dạng chung của hợp chất gồm 2 nguyên tố hóa học được viết như thế nào
Answers
Answer:
What?
Explanation:
Predict the products of the following reactions. (a) sec-butyl isopropyl ether + concd. HBr, heat (c) di-n-butyl ether + hot concd. NaOH (e) ethoxybenzene + concd. HI, heat (g) trans-2,3-epoxyoctane + H+, H2O (b) 2-ethoxy-2-methylpentane + concd. HBr, heat (d) di-n-butyl ether + Na metal (f) 1,2-epoxyhexane + H+, CH3OH (h) propylene oxide + methylamine (CH3NH2) (j) < (1) PhLi phenyllithium (2) H30+ (i) potassium tert-butoxide + n-butyl bromide mCPBA, CH2Cl2 HBr (tm) Yo Ch,0".CH,0H CH20%, CH2OH CH,OH, H+
Answers
The prediction of the products following reactions are as follows:
(a) sec-butyl isopropyl \(ether +\)concd. HBr,\(heat → sec-butyl bromide\)+ isopropanol
(c) di-n-butyl\(ether +\) hot concd. \(NaOH → 2 n-butanol\)+ sodium oxide
(e) ethoxybenzene + concd. HI, \(heat → iodobenzene\)+ ethanol
(g) \(trans-2,3-epoxyoctane + H+\),\(H2O → trans\)-2,3-dihydroxyoctane
(b) 2-ethoxy-2-methylpentane\(+ concd.\) HBr, \(heat → 2-bromo\)-2-methylpentane + ethanol
(d) di-n-butyl \(ether + Na\) \(metal → 2 n-butyl sodium\) + ethane
(f) 1,2\(-epoxyhexane + H+\),\(CH3OH → 1,2-methoxyhexane\)
(h) propylene\(oxide +\)methylamine (CH3NH2) \(→ N-methyl-2-\)propanamine
(j) (1) PhLi phenyllithium (2) \(H30+ → benzene\)\(+ lithium hydroxide\)
(i) potassium \(tert-butoxide + n-butyl\) \(bromide → tert-butyl n-butyl ether\) + potassium bromide
To learn more about ethane, refer below:
https://brainly.com/question/19128101
#SPJ11
07.
Name ONE piece of apparatus used in each of the following separation techniques?
Filtration:..
Evaporation. Evaporating.disn
Sublimation;.
Distillation; Dustallation flash
Decantation:......
Crystallization;
Chromatography:.
Answers
Filtration is a separation method used to separate out pure substances in mixtures comprised of particles some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture.
Evaporation is a technique used to separate out homogeneous mixtures that contain one or more dissolved salts. This process drives liquid components out of solid components. This process usually heats the mixture until the liquid is gone.
Distillation is an effective method of separating mixtures of two or more pure liquids. Distillation is a purification process that vaporizes, condenses, and separates the components of a liquid mixture.
Chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor through a medium in which the components move at different rates. Thin-layer chromatography is a special type of chromatography used for separating and identifying mixtures that are or can be colored especially pigments.
Learn more about Separation techniques here:-https://brainly.com/question/24645716
#SPJ9
please please help me solve this, I have a test and only 20 mins left T-T

Answers
Answer: \(5.6dm^3[/tex[ of gas is produced when 0.1 moles of magnesium nitrate is decomposed.
Explanation:
The balanced chemical equation is:
\(2NH_4NO_3(s)\rightarrow 2MgO(s)+4NO_2(g)+O_2(g)\)
According to stoichiometry :
2 moles of \(NH_4NO_3\) produce = 4 moles of \(NO_2\) gas and 1 mole of \(O_2\) gas
2 moles of \(NH_4NO_3\) produce = 5 moles of gas
Thus 0.1 mole of \(NH_4NO_3\) produce =\(\frac{5}{2}\times 0.1=0.25moles\) of gas
Volume of gas produced = \(moles\times {\text {Molar volume}}=0.25moles\times 22.4dm^3/mol=5.6dm^3\)
Thus \(5.6dm^3[/tex[ of gas is produced when 0.1 moles of magnesium nitrate is decomposed.
If 0.094 moles of magnesium react with excess oxygen, how many moles of magnesium oxide would be formed?
Answers
The equation for this reaction is
\(\text{Mg}+\text{O} \longrightarrow \text{MgO}\)
This means that for every mole of magnesium consumed, 1 mole of magnesium oxide is produced.
So, the answer is 0.094 moles.
What is the pressure in atm of a 5.00 l tank with 5.95 moles of oxygen at 39.3 degrees Celsius
Answers
Answer:
We can use the ideal gas law to solve for the pressure:
PV = nRT
where P is the pressure in atm, V is the volume in liters, n is the number of moles, R is the gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin (K).
First, we need to convert the temperature from Celsius to Kelvin:
T = 39.3 + 273.15
T = 312.45 K
Now we can plug in the values we have:
P(5.00 L) = (5.95 mol)(0.0821 L·atm/mol·K)(312.45 K)
P = (5.95)(0.0821)(312.45) / 5.00
P = 2.98 atm
Therefore, the pressure in the tank is 2.98 atm.
wines with carbon dioxide produced either naturally or mechanically are known as
Answers
Wines with carbon dioxide produced either naturally or mechanically are known as sparkling wines.
Sparkling wines are those that have high levels of carbon dioxide in them.
These wines are fizzy because of the presence of carbon dioxide gas.
When the CO2 gas is dissolved in the wine, it creates bubbles that result in effervescence.
When carbon dioxide is present in wines, either naturally or mechanically produced, the wines are called sparkling wines.
The process of carbonation of wine by nature is known as Methode Ancestrale.
In this process, the carbon dioxide is naturally produced by the yeast during the first fermentation.
In the bottle, this process creates the bubbles.
In contrast, mechanically produced carbon dioxide is created by adding carbon dioxide directly to the wine.
This process is called Charmat Method.
Sparkling wines are perfect for celebrations and special occasions as they add a festive vibe to any event and bring people together.
In conclusion, sparkling wines are wines that have high levels of carbon dioxide in them, either naturally or mechanically produced.
Some popular types of sparkling wines include Champagne, Cava, and Prosecco.
To know more about sparkling wines, visit:
https://brainly.com/question/15073254
#SPJ11
A. If both Assertion & Reason are true and the reason is the correct explanation of the assertion. B. If both Assertion & Reason are true but the reason is not the correct explanation of the assertion. C. If Assertion is true statement but Reason is false. D. If Assertion is false and Reason is true Assertion: .Formation of soluble products from insoluble ones is called precipitation. Reason : Precipitation involves formation of insoluble products from soluble ones
Answers
Answer:
D- Assertion is false and Reason is true
Explanation:
Precipitation is said to have occurred when an insoluble product is formed from soluble ones. Let us take an instance;
BaCl2(aq) + H2SO4(aq) -------> BaSO4(s) + 2HCl(aq)
The barium sulphate so formed is an insoluble product obtained from the reaction of soluble barium chloride and sulphuric acid. This is a typical example of a double replacement reaction as the both ions exchanged partners.
Thus, the assertion is false but the reason is true.
If a compound formula mass was experimentally determined to be 17 AMU the chemical formula would be
Answers
The chemical formula of a compound with a formula mass of 17 will be NH3
Formula massThe formula mass of a compound or molecule is derived by summing the average molar weight of the individual atoms that make up the compound or molecule.
In this case, the formula mass is 17.
For ammonia with chemical formula NH3;
Molar weight of H = 1
Molar weight of N = 14
Formula mass = (14) + (1 x 3)
= 17 AMU
More on formula mass can be found here: https://brainly.com/question/867780
Answer: NH3
Explanation:
how much heat is produced if 7.0 moles of ethane undergo complete combustion?
Answers
The balanced equation for the combustion of ethane, C2H6, is: C2H6 + 3O2 → 2CO2 + 3H2OTo determine how much heat is produced if 7.0 moles of ethane undergo complete combustion, we need to use the balanced equation and the standard enthalpies of formation of the reactants and products.
The standard enthalpy of formation of a compound is the enthalpy change when one mole of the compound is formed from its constituent elements, with all reactants and products in their standard states (usually at 1 atm and 25°C).The standard enthalpies of formation of the reactants and products in the combustion of ethane are:
ΔHf°(C2H6) = -84.68 kJ/mol
ΔHf°(O2) = 0 kJ/mol
ΔHf°(CO2) = -393.51 kJ/mol
ΔHf°(H2O) = -285.83 kJ/mol
Now we can calculate the heat produced by using the difference between the enthalpies of the products and reactants:
2CO2 + 3H2O - (C2H6 + 3O2)
ΔH = 2(-393.51 kJ/mol) + 3(-285.83 kJ/mol) - (-84.68 kJ/mol + 3(0 kJ/mol))
ΔH = -1560.78 kJ/mol
Therefore, if 7.0 moles of ethane undergo complete combustion, the amount of heat produced will be:
-1560.78 kJ/mol x 7.0 mol
= -10,925.46 kJ or -10,925,460 J.
Note that the negative sign indicates that heat is released by the reaction, which is exothermic.
To know more about exothermic visit
https://brainly.com/question/4345448
#SPJ11
The pace huttle orbiter utilize the oxidation of methyl hydrazine by dinitrogen tetroxide for propulion. 4 N2H3CH3(l) 5 N2O4(l) 12 H2O(g) 9 N2(g) 4 CO2(g)
Calculate ΔH° for thi reaction
Answers
The reaction's electron gain enthalopy is ΔH = -5591 kJ/ mol.(approximately)
What, using an example, is electron gain enthalpy?An illustration of electron gain enthalpy ,As a result, an element's second electron gain entropy is positive. For instance, energy is released whenever an electron is attached to a sulfur atom to create an S- ion, whereas energy is needed whenever an electron is attached to an S- ion to create an S2- ion.
Briefing:5N2O4 (l) + 4N2H3CH3 (l) ⇒ 12H2O (g) + 9N2 (g) + 4CO2 (g)
First, sketch the remaining product structures so that you can see the bonds. You ought to purchase the following:
H2O: H-O-H (pretend it's bent)
N2: N≡N
CO2: O=C=O
Step two is to research the average bond strengths of each component (reactants/productsbonds. )'s
Reactants:
N-N: 160 kJ/mol
N=O: 607 kJ/mol
N-O: 210 kJ/mol
N-H: 391 kJ/mol
C-N: 305 kJ/mol
C-H: 413 kJ/mol
Products:
O-H: 467 kJ/mol
N≡N: 941 kJ/mol
C=O: 799 kJ/mol (use the value with * since the bond is in CO2)
Step three is to count the quantity of each type of bond present in the reactants and products. The total bond strengths for every type of bond can then be determined by multiplying those values by the numbers in the balanced chemical equation above.
(For example, since the reactants include a total of nine N-N bonds—five from N2O4 and four from N2H3CH3—you multiply the covalent bond for N-N by nine, or nine times 160, to obtain a total of 1440 KJ/mol.)
Reactants:
N-N: 9*(160)= 1440 kJ/mol
N=O: 10*(607)= 6070 kJ/mol
N-O: 10*(210)= 2100 kJ/mol
N-H: 12*(391)= 4692 kJ/mol
C-N: 4*(305)= 1220 kJ/mol
C-H: 12*(413)= 4956 kJ/mol
Products:
O-H: 24*(467)= 11208 kJ/mol
N≡N: 9*(941)= 8469 kJ/mol
C=O: 8*(799)= 6392 kJ/mol
NOTE: In the balancing equation, I increased the binding energy for the two O-H bonds even by 12 H2O to get 24* for molecules like H2O. (467).
Add all the numbers for the reactants and the products respectively, in step four.
Reactants:
1440+6070+2100+4692+1220+4956= 20478 kJ/mol
Products:
11208+8469+6392= 26069 kJ/mol
In order to find ΔH in step five, remove the bond strengths for the product from the reactants.
ΔH= 20478 - 26069= -5591 kJ/ mol of rxn
To know more about electron gain enthalopy visit :
https://brainly.com/question/28391213
#SPJ4
Which of the following is characteristic of non-metals?
A)
They're always solids at room temperature.
B)
They're strong conductors of electricity.
C)
They tend to be denser than metals.
D)
They tend to gain electrons in chemical reactions.
Answers
Answer:
B
Explanation:
Non metals do not conduct electricity
Answer:
D.
Explanation:
I saw it in the textbook
how do i find the name of an element
Answers
Answer:
The periodic table will say it underneath the symbol.
Explanation:
I don't know how else to describe it but I hope this helps. :)
So 28 (NI) is nickle bc thats what is says on the bottom left

Burning Fuel. 100ml of water Empty cane = 9.33g Fuel Fuel sample initial mass, units(g) Fuel sample final mass, units (g) Water mass, units(g) Initial temperature, t1, units(C) Final (max) temperature, t2, units(C) Ethanol Burner 72.96 71.39 98.87 22 60 Candle 7.85 7.49 98.87 21 40 temperature change T for ethanol burner = final - initial = 60-22 = 38 temperature change T for candle = final - initial = 40-21 = 19 1) calculate the heat absorbed by the water, q, using the equation q = Cp•m•∆t, where q is heat, Cp is the specific heat capacity, m is the mass of water (not of the fuel sample, it is water whose change of temperature you were recording), and ∆t is the change in temperature. For water, Cp is 4.18 J/g•K. Convert your final answer to kJ. This heat was transferred to the water from the burnt fuel. Calculate the energy content (in kJ/g) of each fuel sample (divide kJ by the final-initial mass of the food in g). Finally, calculate the %efficiency in both trials of the experiment. The accepted heat of combustion of paraffin is 41.5 kJ/g, and for ethanol the value is 30.0 kJ/g. Fill in the table with the values you obtained. Fuel Heat produced, J Heat Produced, kJ Energy Content, kJ/g %efficiency name include calculations include calculations name What is the Fuel with the highest energy content? And why?
Answers
The fuel with the highest energy content is the ethanol burner with an energy content of 251.24 kJ/g. This is because ethanol has a higher heat of combustion than paraffin, and the ethanol burner had a higher %efficiency than the candle.
To calculate the heat absorbed by the water, we can use the formula q = Cp•m•∆t, where q is the heat, Cp is the specific heat capacity of water (4.18 J/g•K), m is the mass of water (98.87 g), and ∆t is the change in temperature.
For the ethanol burner, q = (4.18 J/g•K) • (98.87 g) • (38°C) = 15,760.1 J or 15.76 kJ.
For the candle, q = (4.18 J/g•K) • (98.87 g) • (19°C) = 7,781.5 J or 7.78 kJ.
To calculate the energy content (in kJ/g) of each fuel sample, we need to divide the heat produced by the difference in mass of the fuel sample before and after burning.
For the ethanol burner, the energy content = (15.76 kJ) / (72.96 g - 71.39 g) = 251.24 kJ/g.
For the candle, the energy content = (7.78 kJ) / (7.85 g - 7.49 g) = 18.69 kJ/g.
To calculate the %efficiency, we need to use the formula %efficiency = (energy content of fuel sample / accepted heat of combustion of the fuel) x 100%.
For the ethanol burner, %efficiency = (251.24 kJ/g / 30.0 kJ/g) x 100% = 837.47%.
For the candle, %efficiency = (18.69 kJ/g / 41.5 kJ/g) x 100% = 44.99%.
Therefore, the fuel with the highest energy content is the ethanol burner with an energy content of 251.24 kJ/g. This is because ethanol has a higher heat of combustion than paraffin, and the ethanol burner had a higher %efficiency than the candle.
The %efficiency of the ethanol burner is unusually high (837.47%) and is likely due to experimental errors in the measurements. It is important to repeat the experiment multiple times and calculate an average %efficiency to obtain more accurate results.
Learn more about energy content of fuel at : https://brainly.com/question/22764
#SPJ11