How to set up the rate expressions for the following mechanism?
A → B k1
B → A k2
B+C → D k3
If the concentration of B is small compared with the concentrations of A, C, and D, the steady-state approximation may be used to derive the rate law. Derive the rate law, and show that this reaction may follow the first-order equation at high pressures and the second-order equation at low pressures.
Answers
Answer:
Explanation:
From the given information:
A → B k₁
B → A k₂
B + C → D k₃
The rate law = \(\dfrac{d[D]}{dt}=k_3[B][C] --- (1)\)
\(\dfrac{d[B]}{dt}=k[A] -k_2[B] -k_3[B][C]\)
Using steady-state approximation;
\(\dfrac{d[B]}{dt}=0\)
\(k_1[A]-k_2[B]-k_3[B][C] = 0\)
\([B] = \dfrac{k_1[A]}{k_2+k_3[C]}\)
From equation (1), we have:
\(\mathbf{\dfrac{d[D]}{dt}= \dfrac{k_3k_1[A][C]}{k_2+k_3[C]}}\)
when the pressure is high;
k₂ << k₃[C]
\(\dfrac{d[D]}{dt} = \dfrac{k_3k_1[A][C]}{k_3[C]}= k_1A \ \ \text{first order}\)
k₂ >> k₃[C]
\(\dfrac{d[D]}{dt} = \dfrac{k_3k_1[A][C]}{k_2}= \dfrac{k_1k_3}{k_2}[A][C] \ \ \text{second order}\)
Related Questions
Which of the following statements would be an argument against multiculturalism?
(Click all that apply.)
A) Canadians are a mixture of people from various cultural, religious, racial, and ethnic origins. This mixture helps in relations with other countries.
B) Many cultural and ethnic groups have helped build Canada. We should encourage people to preserve their heritage; their traditions are part of Canadian history.
C) Canadians will learn tolerance through being in contact with other cultures. Multiculturalism can help to eliminate discrimination and prejudice.
D) The Canadian government should not spend citizens' tax dollars on multiculturalism. Our taxes are already too high.
Answers
Answer:
D
Explanation:
Maintain the creaminess of your ice cream products! We
here at Creamy Transport ensure that your ice cream
products are transported safely to their destination with
the quality intact. Our mobile storage maintains an ice
cream-friendly temperature of -18°C. No fluctuations
and no melting! We maintain ice cream crystals at their
creamiest size of 15 um. You and your ice cream delights
will be delighted by our service!
You found several studies related to the service
provided by the company. Which data will you use to
make an informed decision? Check all that apply.
data from a celebrity magazine
data verified by other studies
data provided by an ice cream manufacturer
data gathered once to show the reliability of the
company
data from a report put out by a government agency
Lo
Answers
Answer:
Its actually a,b,e
Explanation:
i took the assignment
The data we use to informed decision is data gathered from celebrity magazine, data verified by other studies and data from the report put out by government agencies.
What is data?Data can be defined as collection of information or values that can produce an understandable relationship with the observer and computed phenomenon during analysis or experiments .
Big data can be defined as the process of analysis of high volume and velocity data by advance computational method that produce a understandable results
Thus, the data we use to informed decision is data gathered from celebrity magazine, data verified by other studies and data from the report put out by government agencies.
To learn more about data, refer to the link below:
https://brainly.com/question/10980404
#SPJ2
Fluorine-18 is produced by reacting oxygen-18 with a proton, 11p. The products of this reaction are fluorine-18, a neutron, and a gamma ray. Write a nuclear equation for the production of fluorine-18.
Answers
₈O¹⁸ + ₁¹H(proton) ⇒ ₉F¹⁸ + ₀n¹(neutron) + ₀γ⁰ (gamma)
Further explanationGiven
Fluorine-18
Oxygen-18
Required
Nuclear equation
Solution
Radioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
alpha α particles ₂He⁴ beta β ₋₁e⁰ particles gamma particles ₀γ⁰ positron particles ₁e⁰ neutron ₀n¹The principle used is the sum of the atomic number and mass number before and after the decay reaction is the same
The reaction
₈O¹⁸ + ₁¹H(proton) ⇒ ₉F¹⁸ + ₀n¹(neutron) + ₀γ⁰ (gamma)
\( \frac{2.568 \times 5.8}{4.186} \)
Round off the answer to the correct number of significant figures:
Answers
(2.568 x 5.8)/4.186 = 3.5581460…
= 3.56 (3sf)
You didn’t specify the correct number of significant figures needed.
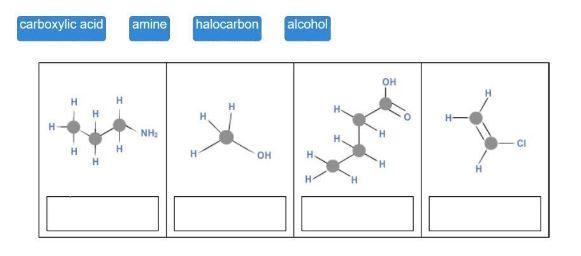
Match each hydrocarbon class to its structure.
4
carboxylic acid
H
H
HT
H
H
H
amine
NH₂
halocarbon
H
OH
alcohol
H.
H
H.
'H
OH
'H
H-
-CI
Answers
The tile's suggested answers include amine, alcohol, carboxyl group, and halocarbon.
Gasoline is it a hydrocarbon?Hydrocarbons are organic substances comprised of hydrogen and carbon, and include petroleum, methane gas, and coal. Alkanes are both a highly combustible chemical and the main source of fuel in the planet. Its uses include diesel, jet fuel, propane, petrol, and petroleum, to name a few.
What makes it a hydrocarbon?The most fundamental category of organic compounds is referred to as a hydrocarbon. As their name implies, they are exclusively made up of the elements hydrogen and carbon. Atoms surround one or more core carbon atoms in hydrocarbon molecules, which are branching or chain-like in shape.
To know more about Hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ1
The complete question is-
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol.
The limiting reactant in a chemical reaction is the reactant that
Answers
Answer:
is used
Explanation:
How many moles in 5 grams?
Answers
Answer:
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant
Explanation:
how does adding energy to an atom affect its electrons
Answers
Using dimensional analysis write the dimension of pressure with its unit
Answers
The unit commonly used to measure pressure is the Pascal (Pa), which is equivalent to a force of 1 Newton per square meter: [Pressure] = 1 [kg/s²·m] = 1 Pa
Pressure is defined as the force per unit area. It can be represented using dimensional analysis by considering the fundamental units of force and area. Force is represented by the fundamental unit of mass (kg) multiplied by the fundamental unit of acceleration (m/s²). Therefore, the dimension of force is [kg·m/s²]. Area is represented by the fundamental unit of length (m) squared. Therefore, the dimension of area is [m²]. To determine the dimension of pressure, we divide the dimension of force by the dimension of area: [Pressure] = [Force] / [Area] = [kg·m/s²] / [m²]
Simplifying the expression, we can cancel out the common unit of length:
[Pressure] = [kg/s²·m] The unit commonly used to measure pressure is the Pascal (Pa), which is equivalent to a force of 1 Newton per square meter: [Pressure] = 1 [kg/s²·m] = 1 Pa Therefore, the dimension of pressure is [kg/s²·m] and its unit is the Pascal (Pa).
For more question on pressure
https://brainly.com/question/24719118
#SPJ8
An airplane flew 3043 km from Houston to Seattle in 5.5 hours. What was the average speed, in m/s rounded to the nearest hundredth, of the airplane from Houston to Seattle?
Answers
An airplane flew 3043 km from Houston to Seattle in 5.5 hours. What was the average speed, in m/s rounded to the nearest hundredth, of the airplane from Houston to Seattle?
The answer is 553
Answer:
553
Explanation:i did this question today and it was right!
How heavy is a gallon of water
Answers
Answer:
Approximately 8.3 pounds.
Explanation:
Is this correct or does this need a decimal?

Answers
For conversion degree Celsius into kelvin we have to add the value 273 in given value.The temperature in kelvin of 73°C is 346 K.
What is meant by temperature conversion?A temperature converter helps change over the temperature between Fahrenheit and Celsius scale. Temperature is measured using a thermometer.
While Kelvin (K) is the SI unit of temperature, people generally use Centigrade or Celsius (°C) and Fahrenheit (°F) to measure temperature.
For conversion degree Celsius into kelvin we have to add the value 273 in given value.
We have to convert 73°C into kelvin.
Therefore,
= 73 + 273
= 346 Kelvin
Thus, The temperature in kelvin of 73°C is 346 K.
To learn more about the temperature, follow the link;
https://brainly.com/question/11464844
#SPJ1
which is an example of a colloid? a mixture that settles out, a mixture that scatters light, a mixture that is separated by filtration, or a salt and water mixture?
Answers
These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Option B)
A colloid is a type of mixture in which particles are dispersed throughout a medium, creating a homogeneous appearance. Unlike solutions, where the particles are completely dissolved, and suspensions, where the particles settle out, colloids have particles that are larger than those in solutions but smaller than those in suspensions. One characteristic of colloids is that they can scatter light due to the size of the particles. This scattering of light is known as the Tyndall effect. Examples of colloids include milk, fog, and aerosol sprays. These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Therefore option B) is correct
For more question on mixture
https://brainly.com/question/24647756
#SPJ8
Note Complete Question
which is an example of a colloid?
a mixture that settles out,
b mixture that scatters light,
c mixture that is separated by filtration,
d salt and water mixture?
20 points!!!!!!!!UNIT 3 LAB The Art Forger Who Tricked the Nazis Where did the trial take place? What was the defendant accused of? What was strange about his defense? How did Han van Meegeren manage to forge the works of art so well? What did he do to make them look authentic? How could forensic testing have changed this case? What ultimately happened to van Meegeren? Money to Run, But No Skills to Hide How did Schrenker try to fake his own death? How did he get caught? Why is creating a new state ID harder to do these days? Why is it so difficult to fake a passport? What is the easiest way for criminals to obtain a passport? Why does Mr. Abagnale claim it is easy to get a fraudulent passport? What steps does someone have to take to make this happen? Why was Mr. Abagnale arrested? What happened to him after his arrest?
Answers
The Art Forger Who Tricked the Nazis:
The trial took place in Dutch.The defendant was accused of forging art.He admitted to his crime.Han van Meegeren studied the old masters.He made paint mixtures using the pigments and oil from the era of each artist.The use of resins to prove forgery.He was released at first and later jailed for 1 year for selling the paintings to the Nazis.What are the details of Money to Run, But No Skills to Hide?Schrenker crashed his plane and made a fake call. He mail his friend. It is harder to create a fake ID now because of increased security features. With all of the security features, it is not an easy thing to master. To take a legitimate one.
Mr. Abagnale believes that all that is required to obtain a birth certificate is a child's death record from a courthouse. A birth certificate and some white-out on an apartment lease gets you a driver's license, which gets you a passport.
He despised what he was doing and became lonely. He was eventually imprisoned for everything.
Learn more on Mr. Abagnale here: https://brainly.com/question/25189801
#SPJ1
The compound butanol has the following structural formula.
A string of 4 C atoms are bonded above, left, and below to H. The right-hand end is bonded to O, which in turn is bonded to H.
Which of these is a structural isomer of butanol?
A string of 4 C atoms are bonded above, below, left and right to H.
A string of 4 C atoms is bonded above, below, left, and right to H, except the second C, which is bonded below to O, which is bonded below to H.
A string of 4 C atoms is bonded above, below, left, and right to H, but the chain is interrupted between the first and second C, which are bonded to an O between them.
A string of 4 C atoms is bonded above, below, and left to H, except the last C has no H below and is double-bonded to an O to the right.
Answers
The structural formula of butanol is C4H9OH. It consists of a chain of four carbon atoms, with a hydroxyl (-OH) group attached to one of the carbon atoms. Butanol has several structural isomers, which have the same molecular formula but different structural formulas.
A structural isomer is a compound that has the same molecular formula as another compound but has a different arrangement of its atoms. A string of 4 C atoms are bonded above, below, left, and right to H, except the second C, which is bonded below to O, which is bonded below to H is a structural isomer of butanol.
This is called butan-2-ol. The structural formula of butan-2-ol is CH3CH(OH)CH2CH3. In this isomer, the hydroxyl group is attached to the second carbon atom in the chain, whereas in butanol, the hydroxyl group is attached to the first carbon atom in the chain.
For more question on atoms
https://brainly.com/question/6258301
#SPJ8
After 56.0 min, 40.0% of a compound has decomposed. What is the half‑life of this reaction assuming first‑order kinetics?
Answers
Answer:
Go ahead and plug in the percentages and time to find the answer.
Explanation:
The amount of a substance with half-life h, that remains after time t is 0.5t/h
Since 26% has decomposed, 74% remains.
So .74 = 0.580/h
ln .74 = (80/h) ln 0.5
h/80 = ln 0.5 / ln .74
h = 80 ln 0.5 / ln .74
h = 184.16 minutes
A substance, W has a concentration of 0.02mol when its molar mass was found to be
74.0 gmol-1 . Another substance V contains 1.00x1023 atoms and has molar mass of
40.0gmol-1. Which of the two substances has the greater mass (in grams)? [L =
6.02x1023]
ii) A 250 cm3
solution contains 14.63g of sodium chloride (NaCl). Calculate the
concentration of the solution in moldm-3
[Na= 23, Cl = 35.5]
Answers
Answer:
Explanation:
mass of W in gram = mole x molecular weight
= .02 x 74 = 1.48 gm
mass of V in gram
first of all we shall calculate the no of moles of V
1 mole = 6.0 x 10²³ atoms
1 x 10²³ atoms = 1 / 6 moles
mass of V in grams
= 40 / 6
= 6.67 grams .
So V has greater mass .
ii )
molecular weight of sodium chloride
= 58.5 gm
14.63 gram of sodium chloride
= 14.63 / 58.5 = .25 moles
250 cm³ = 250 x 10⁻³ dm³
So 250 x 10⁻³ dm³ of solution contains .25 moles of salt
1 dm³ of solution will contain .25 / 250 x 10⁻³ mole
= 1 mole
so concentration of solution is 1 mole per dm³
A piece of metal weighing 59.0 g was heated to 100 C and then put into 100.0 g of water (initially at 23.0 C) the metal and water were allowed to come to an equilibrium temperature, determined to be 27.5 C. Assuming no heat lost to the environment, calculate the specific heat of the metal.

Answers
We have a hot piece of metal that is put in water, and the metal and water are allowed to come to an equilibrium. We can consider that no heat is lost. So the amount of heat that the piece of metal is losing, is gained by the water. The piece of metal is heating the water. We can write that as:
Q water = - Q metal
Then the general formula for the heat of anything is:
Q = m * C * ΔT
So:
Qwater = - Qmetal
mw * Cw ΔTw = -
How much 0.160 M HC1 is required to completely neutralize 20.0 mL of 0.270 M NaOH?
Answers
Answer:
Explanations:
Ammonia, NH3, and oxygen can be reacted together in the presence of a catalyst to form only nitrogen monoxide and water. The number of moles of oxygen consumed for every 15.0 moles of NO produced is:___________.
Answers
Answer:
18.75 moles of O2.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
NH3 + O2 —> NO + H2O
There are 3 atoms of H on the left side and 2 atoms on the right side. It can be balance by putting 4 in front of NH3 and 6 in front of H2O as shown below:
4NH3 + O2 —> NO + 6H2O
There are 4 atoms of N on the left side and 1 atom on the right side. It can be balance by putting 4 in front of NO as shown below:
4NH3 + O2 —> 4NO + 6H2O
Now, there are a total of 10 atoms of O on the right side and 2 atoms on the left side. It can be balance by putting 5 in front of O2 as shown below:
4NH3 + 5O2 —> 4NO + 6H2O
Now the equation is balanced.
Now, we can determine the number of moles of oxygen consumed for every 15.0 moles of NO produced as follow:
4NH3 + 5O2 —> 4NO + 6H2O
From the balanced equation above,
5 moles of O2 were consumed to produce 4 moles of NO.
Therefore, Xmol of O2 will be consume to produce 15 moles of NO i.e
Xmol of O2 = (5 x 15)/4
Xmol of O2 = 18.75 moles.
Therefore, 18.75 moles of O2 is consumed for every 15 moles of NO produced.
using the partial charges, draw out the full reaction mechanism for this acid-base reaction: draw the arrows to show how the arrows to show how the electrons flow from source to sink.
Answers
In an acid-base reaction, the partial charges on the molecules are used to determine the flow of electrons from one atom to another. The partial charges indicate where the electrons are more or less likely to be found, and this helps to determine the direction of the electron flow.
In the reaction mechanism, the arrows are drawn from the atom with the partial negative charge (the electron source) to the atom with the partial positive charge (the electron sink). This indicates the flow of electrons from the source to the sink.
For example, in the reaction between a hydrogen ion (H+) and a hydroxide ion (OH-), the partial charges are as follows:
H+ has a partial positive charge and OH- has a partial negative charge. The arrow is drawn from the oxygen atom in the hydroxide ion (the electron source) to the hydrogen ion (the electron sink). This indicates that the electrons are flowing from the oxygen atom to the hydrogen ion.
Overall, the partial charges are used to determine the direction of the electron flow in an acid-base reaction, and the arrows are drawn to show this flow from the electron source to the electron sink.
Know more about electrons
https://brainly.com/question/25674345
#SPJ11
4. Long answer type questions: a. b. C. d. e. f. g. h. j. i. What are the constituent gases of air? Why is the surrounding air not seen with the eyes? How do you prove that air supports burning? How do you show that air occupies space? How do you prove that air has weight? How is air useful to us? Mention any three points. Write any three properties of air. How can you say that air exerts force? Write any four effects of air pollution. Write any three causes of air pollution and any two control measures of it.
Answers
1. The constituent gases of air are:
Nitrogen Oxygen Argon Carbon Dioxide2. The surrounding air is not seen with the eyes because it is transparent. Air molecules are not visible to the na-ked eye, and they do not scatter or absorb visible light significantly. Therefore, air appears colorless and transparent.
What is air?3. To prove that air supports burning, you can perform an experiment with a burning candle. Place a glass jar or bell jar over a lit candle, ensuring that the jar is airtight. As the candle burns, it consumes oxygen from the air inside the jar. Eventually, the candle flame will go out due to the lack of oxygen, proving that air (specifically oxygen) is necessary for burning.
4. To show that air occupies space, you can perform a simple experiment using a plastic bottle or syringe. Fill the bottle or syringe with water, ensuring there are no air bubbles. Then, cover the opening tightly and try to compress the air inside. You will find that it is not possible to compress the air significantly, indicating that air occupies space.
5. To prove that air has weight, you can use a sensitive balance or scale. Weigh an airtight container or balloon, and then fill it with air. The weight of the container or balloon with the added air will be greater than its initial weight, demonstrating that air has weight.
6. Air is useful to us in various ways. Three points highlighting the importance of air are:
Breathing and RespirationCombustion and Energy ProductionClimate Regulation7. Three properties of air include:
Air is Compressible: Air can be compressed or expanded under different conditions, allowing it to fill various spaces and containers.Air has Mass: Air molecules have mass, which means air itself has weight. It exerts pressure on objects and surfaces.Air Exerts Pressure: Due to the collisions of air molecules with surfaces, air exerts pressure in all directions. This pressure is known as atmospheric pressure.Air exerts force in various ways. For example, air pressure allows objects like airplanes to fly by providing lift. Air resistance or drag opposes the motion of objects moving through the air, creating a force that can affect their speed and trajectory.
8. Four effects of air pollution include:
Respiratory ProblemsEnvironmental Damage:Climate ChangeHuman Health Impacts9. Causes of pollution:
Industrial EmissionsVehicle EmissionsResidential and Agricultural Activities10. Two control measures for air pollution include:
Emission ReductionAir Quality RegulationsLearn more about air on https://brainly.com/question/15215203
#SPJ1
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
Hydrogen gas is collected over water at 23°C, 767 torr. At this temperature the vapor pressure of water is 21.0 torr. What is the partial pressure of hydrogen in the collected gas?
Answers
The partial pressure of hydrogen gas in the collected gas is 746 torr.
To determine the partial pressure of hydrogen gas in the collected gas, we need to consider the difference between the total pressure of the collected gas and the vapor pressure of water at the given temperature. The partial pressure of hydrogen gas is the pressure exerted by hydrogen alone.
Given information:
Total pressure of the collected gas (Ptotal) = 767 torr
Vapor pressure of water (Pwater) = 21.0 torr
The partial pressure of hydrogen gas (Phydrogen) can be calculated using Dalton's law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas.
Phydrogen = Ptotal - Pwater
Plugging in the given values:
Phydrogen = 767 torr - 21.0 torr
Phydrogen = 746 torr
Therefore, the partial pressure of hydrogen gas in the collected gas is 746 torr.
It's important to note that in this calculation, we assume that the water vapor does not react with or dissolve in the hydrogen gas and that the gases behave ideally. Additionally, it's assumed that the collected gas is dry, meaning all the water vapor has been removed or does not significantly contribute to the total pressure.
Fo rmore such questions on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
1. How is the law of conservation of mass shown by a balanced chemical equation?
A) The subscripts must be the same on both sides of the equation.
B) The total volumes of the substances must be the same on both sides of the equation.
C) The coefficients must be the same on both sides of the equation.
D) The number of each type of atom must be the same on both sides of the equation.
Answers
Answer:
D
Explanation:
hope this helps
explain how the particles of a substance change as a substance changes from a solid to a liquid to a gas.
Answers
Answer:
Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the sur- face particles of the solid gain enough energy to become a gas.When dry ice becomes a gas, it absorbs thermal energy from water vapor in the air.
Explanation:
if you have 58 g of Copper what is the mass of Silver nitrate?
____Cu +____AgNO3 →
____Ag+ ____Cu(NO3)2
Answers
Answer:
Add 2 to Cu in first part of problem only
select the best benefit of the text structure of the lewis and clark journal entries. the compare and contrast structure of the text allows the reader to evaluate the experience both men had on their expedition. the cause and effect structure of the text allows the reader to see how the men's decisions greatly impacted their traveling companions. the chronological structure of the text allows the reader to follow their expedition to see how their journey unfolded. the description structure of the text allows the reader to gain a real appreciation for each place the explorers visited, so it may inspire them to visit those places as well.
Answers
The best benefit of the text structure of the Lewis and Clark journal entries is C. The chronological structure of the text allows the reader to follow their expedition to see how their journey unfolded.
What is chronological structure?The art or process of placing events or occurrences in chronological order is known as chronology. Typically, it goes from first to last, or from the earliest event to the most recent events.
In the case of a degree certificate holder, for instance, the chronological order of their education is from primary school, which is the oldest certificate, to secondary school, and finally tertiary institution.
Most expository writing, which is a type of writing that narrates, describes, informs, or explains a process, uses chronological order. Place the events in chronological order so that they appear as they did in reality or as they will if you are giving instructions.
In this case, it should be noted that the journal was arranged accordingly as it happened. Therefore, the correct option is C.
Learn more about structures on:
https://brainly.com/question/26719078
#SPJ1
Clark, May 14, 1804]
Monday May 14th 1804 Rained the forepart of the day I determined to go as far as St. Charles a french Village 7 Leags. up the Missourie, and wait at that place untill Capt. Lewis Could finish the business in which he was obliged to attend to at St Louis and join me by Land from that place 24 miles; by this movement I calculated that if any alterations in the loading of the Vestles or other Changes necessary, that they might be made at St. Charles I Set out at 4 oClock P.M.
[Lewis, May 15, 1804]
Tuesday May 15th It rained during the greater part of last night and continued untill 7 OCk. A.M. after which the Prarty proceeded, passed two Islands and incamped on the Stard. shore at Mr. Fifer's landing opposite an Island, the evening was fair.
select the best benefit of the text structure of the lewis and clark journal entries. the compare and contrast structure of the text allows the reader to evaluate the experience both men had on their expedition. the cause and effect structure of the text allows the reader to see how the men's decisions greatly impacted their traveling companions. the chronological structure of the text allows the reader to follow their expedition to see how their journey unfolded. the description structure of the text allows the reader to gain a real appreciation for each place the explorers visited, so it may inspire them to visit those places as well
which of the following is a fission reaction?
carbon-12 and hydrogen-1 combining to form a nitrogen-13 atom
Answers
Answer:The reactions are as follows: a carbon-12 (12C) nucleus captures a hydrogen nucleus (1H, a proton) to form a nucleus of nitrogen-13 (13N); a gamma ray (γ) is emitted in the process. The nitrogen-13 nucleus emits a positive electron (positron, e+) and becomes carbon-13 (13C).
What is a statistical sample? Why is it important to have a large sample size?
Answers
A sample is a set of people or objects collected form a statistical population by a defined procedure
It is important to have a large sample size for research, larger sample size is provide more accurate results in a experiment
Hope this answer helps