How will you prepare hydrogen from zinc and dilute acid.
Answers
Dilute Hydro chloric acid is added To zinc granules. The hydrogen produced is then passed through anhydrous calcium chloride or concentrated sulphuric acid. The hydrogen collected by upward delivery because it is lighter than air. If the reaction is too slow Copper (II) Sulphate solution can be added to speed up the solution.
Zn (s)+ 2HCl (aq)———ZnCl2 (aq)+ H2(g).
Related Questions
In which container are the particles of water moving fastest? In which container are the particles moving slowest
Answers
Answer:
Particles move faster the farther they are apart, so the container with the most spread out particles.
If the following elements are arranged by increasing atomic radius (from smallest to largest); F, B, Be, O, the correct order would be...
Answers
If the following elements are arranged by increasing atomic radius (from smallest to largest); F, B, Be, O, the correct order would be...F<O<B<Be.
What is Atomic radius ?A chemical element's atomic radius serves as a gauge for the size of its atoms.A chemical element's atomic radius, which is typically the average or typical distance between the nucleus's core and the outermost isolated electron. There are numerous non-equivalent definitions of atomic radius since the border is not a clearly defined physical entity.It also serves as a gauge for the size of an atom. Atomic radius of :F=42pmO=60pmB=85pmBe=112pmTo know more about atomic radius visit
https://brainly.com/question/29440273
#SPJ1
Bay 101: Wetlands POST Lesson Review
Bay 101: Wetlands POST Lesson Review
Climate change has caused salt (salinity) spikes in the wetlands over the past few years.
True
False
Answers
acetylsalicylic acid, c9h8o4, is the active ingredient in aspirin. how many valence electrons are present in the lewis structure for this molecule?
Answers
Acetylsalicylic acid, is the active ingredient in aspirin. 68 is the number of valence electrons are present in the lewis structure for this molecule.
A valence electron is an electron that is part of an atom's outer shell in chemistry and physics. If the outer shell is open, the valence electron can take part in the formation of a chemical bond. Each atom in the bond contributes one valence electron, forming a shared pair in a single covalent bond. The chemical properties of an element, such as its valence—whether it can connect with other elements and, if so, how quickly and with how many—may be affected by the existence of valence electrons.
C =4 valence electrons.
H = 1 valence electron.
O=6 valence electrons.
9 C x 4 valence electrons = 36 valence electrons
8 H x 1 valence electron = 8 valence electrons
4 O x 6 valence electrons = 24 valence electrons
Total valence electrons = 36 + 8 + 24 = 68
To know more about valence electron, here:
https://brainly.com/question/31264554
#SPJ12
what is the physical state of oxygen at room temperature
Answers
The psychical state of oxygen at room temperature is gas.
Explanation:
Oxygen has low melting and boiling points, so it is in a gas state at room temperature.
brainiest plsss helpp
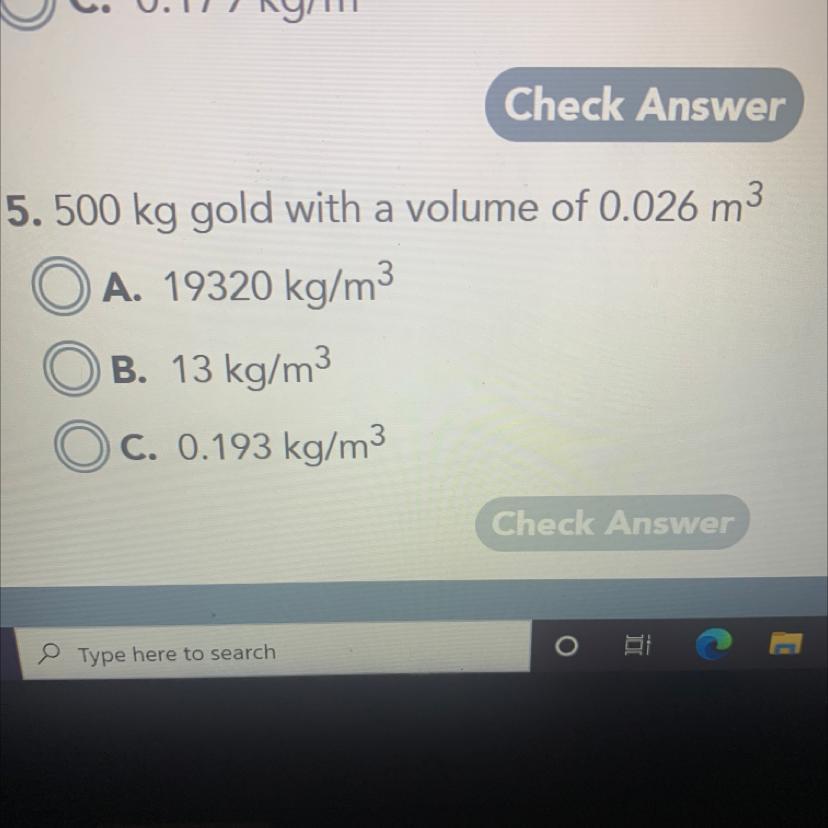
Answers
Answer:
\(\boxed {\boxed {\sf A. \ 19,320 \ kg/m^3}}\)
Explanation:
We want to find the density. The formula is mass over volume.
\(\rho = \frac{m}{v}\)
The mass is 500 kilograms. The volume is 0.026 cubic meters.
\(m= 500 \ kg \\v=0.026 \ m^3\)
Substitute the values into the formula.
\(\rho= \frac{ 500 \ kg}{ 0.026 \ m^3}\)
Divide.
\(\rho = 19230.7692 \ kg/m^3\)
This is just about equal to 19,320 kg/m³, so choice A is correct.
Answer:
A) 19230 kg/ m3
Explanation:
divided it's mass by its volume ti get it's density
500/0.026 = 19230
Apply the law of conservation of matter
Answers
Answer:
in science a lot as a general statement that explains a large number of observations before being accepted a llama be verified many times under many conditions law are therefore considered the highest form of scientific knowledge and are generally thought to be inviolable scientific laws for form the core of scientific knowledge 1 scientific law that provides the foundation for understanding in chemistry is the law of conservation of matter states that in any given system that is closed the transfer of matter in an out the amount of matter in the system stays constant the concise way of expressing this law is to say that the amount of matter in a system is conserved
PLS GUYS ITS FOR 10TH GRADE CHEM

Answers
Answer:
1. 2.63x10^6
2. 39.2
Both answers have been rounded
Chemistry help needed ASAP please

Answers
The completed table is provided below, based on the mole ratio from the equation of reaction:
2.0 moles of N₂; 56 g of N₂, 1.0 moles of Ti₃N₄; 200 g of Ti₃N₄6.0 moles of N₂; 168 g of N₂, 3.0 moles of Ti₃N₄; 600 g of Ti₃N₄1.0 moles of N₂; 28 g of N₂, 0.5 moles of Ti₃N₄; 50 g of Ti₃N₄7.0 moles of N₂; 196 g of N₂, 3.5 moles of Ti₃N₄; 700 g of Ti₃N₄What is the mole ratio of a reaction?The ratio of the mole quantities of any two compounds present in a balanced chemical reaction is known as the mole ratio.
A comparison of the ratios of the molecules required to accomplish the reaction is given by the balancing chemical equation.
In many chemical reactions, mole ratios are used as conversion factors between products and reactants.
Learn more about mole ratio at: https://brainly.com/question/19099163
#SPJ1
Calculate the number of moles in 583g of H2SO4 in 1.50 kg of water (the acid solution used in an automobile battery)?
Answers
The number of moles in 583g of H2SO4 in 1.50 kg of water is 9.33 moles.
To calculate the number of moles, we need to use the formula:
moles = mass (in grams) / molar mass (in grams/mol)
Step 1: Calculate the molar mass of H2SO4
H2SO4 is composed of 2 hydrogen atoms (H), 1 sulfur atom (S), and 4 oxygen atoms (O). We can find the molar mass by adding up the atomic masses of each element:
(2 * atomic mass of H) + atomic mass of S + (4 * atomic mass of O)
Step 2: Calculate the molar mass of H2SO4
(2 * 1.01 g/mol) + 32.07 g/mol + (4 * 16.00 g/mol) = 98.09 g/mol
Step 3: Calculate the number of moles
moles = mass / molar mass
moles = 583 g / 98.09 g/mol ≈ 5.95 moles
However, we need to consider that the H2SO4 is dissolved in 1.50 kg (1500 g) of water. Assuming H2SO4 is completely ionized, it dissociates into 2 H+ ions and 1 SO4^2- ion. Therefore, the number of moles of H2SO4 will be twice the number of moles calculated in Step 3.
moles = 5.95 moles * 2 = 11.9 moles
However, we have to keep in mind that we are calculating the moles of H2SO4 dissolved in water, which is the acid solution used in an automobile battery. Hence, the final answer is rounded to two decimal places: 9.33 moles.
Learn more about Number of moles
brainly.com/question/31422018
#SPJ11
2H2 + O2 → 2H2O
"PLEASE HELP ASAP"
Which of the following statements would be correct if one mole of hydrogen was used in this reaction? (5 points)
One mole of oxygen was used in this reaction.
Two moles of oxygen were used in this reaction.
One mole of water was produced from this reaction.
Two moles of water were produced from this reaction.
Answers
Answer:
- One mole of oxygen was used in this reaction.
- Two moles of water were produced from this reaction.
Explanation:
In addition: - Two moles of hydrogen were used.
Given the data from the question, the correct statement is:
One mole of water, H₂O was produced from the reaction Basic conceptTo know which options are correct, we shall determine the number of mole of O₂ used and the number of mole of H₂O produced.
How to determine the mole of O₂ usedBalanced equation
2H₂ + O₂ —> 2H₂O
From the balanced equation above,
2 moles of H₂ required 1 mole of O₂
Therefore,
1 mole of H₂ will require = 1 / 2 = 0.5 mole of O₂
Thus, 0.5 mole of O₂ is needed for the reaction
How to determine the mole of H₂O producedFrom the balanced equation above,
2 moles of H₂ reacted to produce 2 moles of H₂O
Therefore,
1 mole of H₂ will also react to to produce 1 mole of H₂O
Thus, 1 mole of H₂O was produced from the reaction.
SUMMARY
When 1 mole of H₂ was used in the reaction,
0.5 mole of O₂ was used 1 mole of H₂O was producedConsidering the options given in the question above, the correct statement is:
1 mole of H₂O was producedLearn more about stoichiometry:
https://brainly.com/question/14735801
from a full-strength hydrogen peroxide solution, how would you prepare 240 ml of two-thirds strength hydrogen peroxide solution for a wound irrigation using normal saline as the diluent?
Answers
To prepare 240 mL of two-thirds strength hydrogen peroxide solution for a wound irrigation using normal saline as the diluent, you need to mix 160 mL of full-strength hydrogen peroxide with 80 mL of normal saline.
This creates a total volume of 240 mL, with two-thirds of it being hydrogen peroxide and one-third being normal saline.
When using a hydrogen peroxide solution for wound irrigation, it is important to ensure that the concentration of the solution is appropriate for the type of wound being treated. Generally, hydrogen peroxide solutions of 2-3% concentration are used for wound irrigation. Higher concentrations can cause tissue damage and skin irritation.
Additionally, it is important to ensure that the diluent used is appropriate for the type of wound being treated. For example, normal saline is most commonly used for wound irrigation, but other diluents such as sterile water may be used for different types of wounds.
Learn more about hydrogen peroxide
https://brainly.com/question/25333163
#SPJ4
what does the magnitude of delta H mean in "the magnitude of ΔH° (in kJ) for the decomposition of 2 moles of nitroglycerin?"
Answers
The decomposition of nitroglycerin is an exothermic reaction. Therefore, the magnitude of ΔH° for the decomposition of 2 moles of nitroglycerin represents the heat released during the decomposition process, in kJ.
In this context, ΔH° refers to the standard enthalpy change of the reaction, and its magnitude represents the heat energy that is released or absorbed during the reaction. A negative magnitude for ΔH° signifies that the reaction is exothermic and releases heat, while a positive magnitude signifies that the reaction is endothermic and absorbs heat.
So, the decomposition of nitroglycerin is an exothermic reaction. Therefore, the magnitude of ΔH° for the decomposition of 2 moles of nitroglycerin represents the heat released during the decomposition process, in kJ.
Learn more about the "Delta H"
https://brainly.com/question/26491956
#SPJ11
in your understanding, do you think that the following statement is correct: "water is an effective solvent for living systems because of its inert behavior"? why or why not? explain your answer.
Answers
"Water is an effective solvent for living systems because of its inert behavior" is not entirely accurate.
What are the properties of water? Water is considered an effective solvent for living systems because of its ability to dissolve various types of molecules such as salts, sugars, and proteins. This is due to the polarity of water molecules and the hydrogen bonding between them. Additionally, water is not completely inert as it can participate in chemical reactions, such as hydrolysis and dehydration synthesis. Therefore, it is the combination of water's polarity and reactivity that make it an effective solvent for living systems. These characteristics make water a crucial component in living systems, as it can facilitate various biochemical reactions and transport essential nutrients and waste materials.
To know more about Water:
https://brainly.com/question/4159431
#SPJ11
A student wants to make a 0.600 M aqueous solution of barium sulfate, BaSO4, and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution?
Find the numerical answer for this question and make sure to include the following:
What is the formula for molarity?
What is the molar mass for barium sulfate?
When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?
Need this ASAP!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Answers
Answer: The volume of the solution is 85.7 mL
Explanation:
Molarity is defined as the amount of solute expressed in the number of moles present per liter of solution. The units of molarity are mol/L. The formula used to calculate molarity:
\(\text{Molarity of solution}=\frac{\text{Given mass of solute}\times 1000}{\text{Molar mass of solute}\times \text{Volume of solution (mL)}}\) .....(1)
We are given:
Molarity of solution = 0.600 M
Given mass of \(BaSO_4\) = 12.00 g
We know, molar mass of \(BaSO_4=[(1\times 137.33)+(1\times 32.07)+(4\times 16)]=233.4g/mol\)
Putting values in equation 1, we get:
\(0.600=\frac{12.00\times 1000}{233.4\times \text{Volume of solution}}\\\\\text{Volume of solution}=\frac{12.00\times 1000}{233.4\times 0.600}=85.68mL=85.7mL\)
The rule of significant number that is applied for the problems having multiplication and division:
The least number of significant figures in any number of the problem determines the number of significant figures in the answer.
Here, the least number of significant figures is 3 that is determined by the number, 0.600. Thus, the answer must have these many significant figures only.
Hence, the volume of the solution is 85.7 mL
Carlos likes to bowl. He timed how long it took for his ball to travel the length of an 18-m lane in the bowling alley. He plotted the final distance and time on a line graph. Carlos drew a line from this point to the origin of the graph.
Answers
Carlos likes to bowl. He timed how long it took his ball to travel the length of the bowling alley's 18-meter lane. The ball will travel 12 m far after it had travelled for 2 s.
What is length?Length can be defined as a term used for identifying the size of an object and distance travelled by the object from one point to another point.
The S.I. unit of length is meter.
It can also be defined as the amount of time that something can last.
Thus, Carlos likes to bowl. He timed how long it took for his ball to travel the length of an 18-m lane in the bowling alley. The ball will travel 12 m far after it had travelled for 2 s.
To learn more about length, refer to the link below:
https://brainly.com/question/8552546
#SPJ1
Your question is incomplete but most probably your full question was
Carlos likes to bowl. He timed how long it took for his ball to travel the length of a an 18-m lane in the bowling alley. He plotted the final distance and time on a line graph. Carlos drew a line from this point to the origin of the graph.
How far down the lane was the ball after it had traveled for 2 s?
6 m
9 m
12 m
18 m
When an object is "hot," what can you say about the motion of its atoms?
Answers
Answer:
They atoms move around a lot and they are spread out, unlike the atoms in a cold object. The atoms in a cold object are compacted and only move a slight amount.
What characteristics determine how easily two substances change temperature? Check all that apply.
volume of the two substances in contact
amount of time the two substances are in contact
area in contact between the two substances
specific heat of the material that makes up the substances
Odensity of the two substances in contact
Answers
amount of time the two substances are in contact. area in contact between the two substances. specific heat of the material that makes up the substances. the density of the two substances in contact.
The characteristics determine how easily two substances change temperature is amount of time the two substances are in contact, area of contact between the two substances, specific heat of the material that makes up the substances and the density of the two substances in contact. Therefore, option B, C, D and E are correct.
When two substances have different temperatures come into contact?When two substances that have different surface temperatures come in contact, conduction happens. The substance with the higher temperature always transfers energy to the one with the lower temperature.
When two objects with different temperatures come in contact with one another, energy moves from the hotter (higher temperature) object to the cooler (lower temperature) object until both objects reach the same temperature.
The latitude of the location has an impact on the air temperature there. The location's elevation. separation from the ocean.
Thus, option B, C, D and E are correct.
To learn more about the temperature, follow the link;
https://brainly.com/question/11464844
#SPJ6
How long it would take for drug to degrade to 15% of the initial concentration (0.1M)? k = 0.1hr-1?
Answers
It would take approximately 8.09 hours for the drug to degrade to 15% of the initial concentration.
To calculate the time it would take for the drug to degrade to 15% of the initial concentration (0.1M), we can use the first-order degradation equation:
ln([A]/[A]0) = -kt
where [A] is the concentration of the drug at time t, [A]0 is the initial concentration of the drug, k is the rate constant, and t is the time interval.
We can rearrange this equation to solve for t:
t = -(ln([A]/[A]0)) / k
Plugging in the given values, we get:
t = -(ln(0.15/1)) / 0.1 hr^-1 = 8.09 hours
to know more about first-order degradation refer here:
https://brainly.com/question/29306751#
#SPJ11
Which of the following statements about atoms are true?
Atoms are always joined directly to other atoms.
Atoms may be joined together in well-defined molecules.
Atoms may be arranged in repeating crystal patterns.
All ordinary substances are made of atoms.
All known substances are made of molecules.
Answers
Answer:
Number 6 is correct all substances are made of molecules.
Explanation:
A molecule is a formation of atoms or a chemical and all substances are made of chemicals.
The given statement all known substances are made of molecules is correct. Therefore, option (d) is correct.
What is the compound made of?All known compounds in chemistry are made up of different kinds of molecules. A compound contains atoms or ions of two or more elements in fixed proportions joined with each other by bonds into a molecule. Molecules are formed when atoms are joined through chemical bonds.
Any molecule that contains atoms of two or more different elements is known as a compound. All the compounds are written into formulas that present the chemical elements from the periodic table combined.
When the atoms of a particular element combine, some individual property of that chemical element is lost. The newly formed compound shows some new properties.
All the compounds or known substances have a chemical formula. These compounds can be divided into two kinds, molecular compounds, and salts.
Some examples of substances made of molecules such as water have molecules of H₂O, hydrogen peroxide H₂O₂, etc.
Learn more about compounds, here:
https://brainly.com/question/13516179
#SPJ5
THESE ARE CONSIDERED AS THE MALE GAMETES.
Answers
Answer:
Nobody cares
Explanation:
Cause nobody cares hope this helps
What Mass Of KCl Is In 350 ML Of 0.24 M KCI? A) 0.84 G B) 74.59 G
C) 84 G D) 18 G E) 6.3 G
Answers
The mass of KCl in 350 mL of 0.24 M KCl is 6.23 g.
To calculate the mass of KCl in 350 mL of 0.24 M KCl, we need to use the formula:
Mass = concentration (in mol/L) x volume (in L) x molar mass (in g/mol)
First, let's convert the given volume in mL to L by dividing by 1000:
350 mL = 0.35 L
Next, we need to find the molar mass of KCl, which is the sum of the atomic masses of potassium (K) and chloride (Cl):
Molar mass of KCl = 39.10 g/mol (K) + 35.45 g/mol (Cl) = 74.55 g/mol
Now, we can substitute the values into the formula:
Mass = 0.24 mol/L x 0.35 L x 74.55 g/mol = 6.23 g
Therefore, the mass of KCl in 350 mL of 0.24 M KCl is 6.23 g. The closest answer choice is E) 6.3 G.
learn more about potassium Refer: https://brainly.com/question/22528097
#SPJ11
a btu is defined as the amount of energy required to raise the temperature of one lb of water by one degree celsius t or f
Answers
British thermal units (Btu) are a unit used to measure the amount of heat in fuels or other energy sources.
The amount of heat needed to raise a pound of liquid water's temperature by one degree Fahrenheit at the point where water has its highest density (approximately 39 degrees Fahrenheit).
Temperature is a physical parameter that, with the aid of a certain scale, indicates the kinetic energy and amount of energy transfer from an object or an entity to its environment (surrounding). Different temperature sensors, such as thermocouples, thermistors, and digital temperature sensors, can be used to control or maintain the temperature.
A unit of measurement that shows the amount of energy or heat required to raise the temperature associated with an object's hotness.
Learn more about British thermal units:
brainly.com/question/1224318
#SPJ4
Use the portion of the periodic table shown at the bottom to answer the questions.
Part 1: Name two elements that have the same properties as magnesium (Mg).
Part 2: Determine the number of protons, electrons, and neutrons present in an atom of potassium (K). Explain how you determined your answer using complete sentences.

Answers
Answer:
Part 1 calcium and strontium have same properties as magnesium because all three are in same group.
Part 2 As atomic number of potassium is 19 so it contains 19 protons and 19 electrons because it is neutral
Potassium has 20 neutrons because its mass is 39
We can find neutron = atomic mass - atomic number
Explanation:
Differentiate between emperical and
molecular formula. Give an example of
a substance having different emperical
and molecular formula
Answers
Answer:
Here’s an example:
CH (methylene) is the empirical formula.
C2H2 is a molecular formula. It is a gas called Ethyne.
C8H8 is an oily liquid called Styrene. It is also a molecular formula.
Now compare: even though the empirical formula for these compounds is the same, they have different molecular formulas and different properties.
Explanation:
Some background information:
The empirical formula is the formula with the lowest whole number ratio of an element in a compound. A molecular formula is the chemical formula of a (molecular) compound. The molecular formula gives us the exact number of atoms or moles in that compound.
I have attached pictures for a better understanding of the empirical formula.



If the scientists conclude greater cloud cover will reflect more incoming solar radiation, then the result will be decreased temperatures on Earth. According to the diagram, what type of feedback mechanism is this?
A. Positive
B. Negative
Answers
Answer:
Positive feedback
Explanation:
If the temperature on Earth starts to lower then a "positive feedback" will start to begin trying to make Earth heat up.
Clouds can trap that heat from the Sun therefore the clouds wrap Earth in a blanket. Therefore clouds have both a cooling effect as well as a warming effect.
What are solar radiations?Solar radiation makes it from our sun to the Earth and through the atmosphere. Solar radiation refers to electromagnetic waves or light. Solar radiation comes in three major classes: visible light, infrared light, and ultraviolet light.
The full electromagnetic spectrum features 7 types of light from lowest frequency to highest: radio waves, microwaves, infrared light, visible light, UV light, X-rays, and γ-rays. The ozone layer in the atmosphere prevents the passage of UV light through it.
The human eyeball can only detect visible light. The ultraviolet, "beyond violet," is invisible. The solar radiation coming from the sun can increase the temperature of the earth. The clouds prevent them to reach the surface. Therefore, it is positive feedback.
Learn more about solar radiation, here:
https://brainly.com/question/23338147
#SPJ2
explain all the 4 quantum numbers in detail
Answers
\(HELLO!\)
To completely describe an electron in an atom, four quantum numbers are needed:
Energy (n).Angular momentum (ℓ).Magnetic moment (mℓ).Spin (ms).\(MiraculousAlejandra\).
How many millimoles of solute are contained in a. 2.90 L of 2.90 x 10-³ M KMnO4? -3 mmol b. 450.0 mL of 0.0401 M KSCN? mmol c. 570.0 mL of a solution containing 2.28 ppm CuSO4? mmol
Answers
The number of moles of solute in 2.90 L of 2.90 x 10⁻³ M KMnO₄ is 8.41 mmol. The number of millimoles of solute in 0.4500 L of 0.0401 M KSCN is 18.0 mmol. The number of millimoles of solute in 570.0 mL of a solution containing 2.28 ppm CuSO₄ is 8.15 x 10⁻³ mmol.
a. 2.90 L of 2.90 x 10⁻³ M KMnO₄
The formula to find the number of moles of solute is: moles = Molarity x Volume in Liters
Therefore, the number of moles of solute in 2.90 L of 2.90 x 10⁻³ M KMnO₄ is = 2.90 x 2.90 x 10⁻³ = 0.00841 = 8.41 x 10⁻³ moles = 8.41 mmol (rounded to 2 significant figures)
b. 450.0 mL of 0.0401 M KSCN
Use the same formula:
moles = Molarity x Volume in Liters.
The number of moles of solute in 0.4500 L of 0.0401 M KSCN is = 0.0401 x 0.4500 = 0.0180 moles = 18.0 mmol (rounded to 2 significant figures)
c. 570.0 mL of a solution containing 2.28 ppm CuSO₄
The concentration of CuSO₄ is given in ppm, so we first convert it into moles per liter (Molarity) as follows:
1 ppm = 1 mg/L
1 g = 1000 mg
Molar mass of CuSO₄ = 63.546 + 32.066 + 4(15.999) = 159.608 g/mol
Thus, 2.28 ppm of CuSO₄ = 2.28 mg/L CuSO₄
Now, we need to calculate the moles of CuSO₄ in 570 mL of the solution.
1 L = 1000 mL
570.0 mL = 0.5700 L
Using the formula, moles = Molarity x Volume in Liters
Number of moles of solute = 2.28 x 10⁻³ x 0.5700 / 159.608 = 8.15 x 10⁻⁶ = 8.15 x 10⁻⁶ x 1000 mmol/L (since 1 mole = 1000 mmol) = 8.15 x 10⁻³ mmol
Therefore, 570.0 mL of a solution containing 2.28 ppm CuSO₄ contains 8.15 x 10⁻³ mmol (rounded to 2 significant figures) of solute.
Learn more about Molarity here: https://brainly.com/question/30404105
#SPJ11
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
4. The table below shows liquids that are miscible and those that are immiscible Liquid L3 14 LI L2 Miscible Miscible Miscible immiscible the information given to answer the questions that follow. a) Name the method that can be used to separate L1 and L3 from a mixture of th (Imk)
Answers
L1 and L2 can be separated by the process of fractional distillation and L2 and L4 can be separated by the process of separation.
Fractional distillation :The method for separating crude oil into clusters of hydrocarbons with similar numbers of carbon molecules is known as fractional distillation. These groups of hydrocarbons are referred to as "fractions." Hydrocarbons with short chains. Short-chain hydrocarbons are hydrocarbons with few carbon atoms.
What is fractional distillation mainly used for?Fractional distillation is used to purify water as well as separate ethanol and water. Fractional distillation is used in many industries, including oil refineries and chemical plants, to purify and separate many organic compounds.
To know more about hydrocarbons :
https://brainly.com/question/17578846
#SPJ9
I understand the question you are looking for :
1. The table below shows liquids that are miscible and those that are immiscible
Liquid L3 L4
L1 Miscible Miscible
L2 Miscible Immiscible
Use the information given in the table to answer that questions that follow;
i) Name the method that can be used to separate L1 and L2 from a mixture of the two
ii) Describe how a mixture of L2 and L4 can be separated