Hydrogen bond is not formed by 1) oxygen 2) nitrogen 3) chlorine
PLEASE HELP!!!
i mean which cant form hydrogen bond
Answers
Answer:
Chlorine
Explanation:
Even though chlorine is highly electronegative, the best answer is no, and in this class we will consider chlorine not to form hydrogen bonds (even though it has the same electronegativity as oxygen). This is because chlorine is large and its lone electron is in a diffuse orbital, covering a large area, and thus do not have the high charge density to act as a strong hydrogen bond acceptor. But it does form weak hydrogen bonds in solid crystalline hydrogen chloride at very low temperatures.
Related Questions
()3C− − on reaction with HI gives () − − as
the main products and not () − and −
Answers
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I.
When 3C⁻⁻ is reacted with HI, the reaction product obtained is I⁻⁻⁻ as the main product. The C₂H₅I and H⁻ are not produced in significant quantities and cannot be considered the main product.The 3C⁻⁻ compound reacts with HI in the presence of a solvent to produce hydrogen gas, H⁻, C₂H₅I, and I⁻⁻⁻. The primary product obtained is I⁻⁻⁻ because it is stable and has a higher energy than C₂H₅I and H⁻.However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions. The reaction must be carried out in anhydrous conditions and at a low temperature so that the reaction proceeds in the desired direction.
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I. However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions.
To know more about hydrogen visit:
brainly.com/question/30623765
#SPJ11
The boiling temperature of water decreases by about 3.1 C for each 1000-m rise in altitude. What is the decrease in the boiling temperature in K," F, and R for each 1000−m rise in altitude? The decrease in the bolling temperature in K is The decrease in the bolling temperature in F is The decrease in the boiling temperature in R is
Answers
The decrease in boiling temperature in K is 3.1 K, in °F is approximately 5.58 °F, and in °R is approximately 464.58 °R for each 1000 m rise in altitude.
To convert the decrease in boiling temperature from Celsius (°C) to Kelvin (K), Fahrenheit (°F), and Rankine (°R), we can use the following conversion formulas:
K = °C + 273.15
°F = (°C × 9/5) + 32
°R = °F + 459.67
Given that the decrease in boiling temperature is approximately 3.1 °C for each 1000 m rise in altitude, we can calculate the corresponding values:
Decrease in boiling temperature in K:
ΔT(K) = 3.1 °C
ΔT(K) = 3.1 K (since 1 K = 1 °C)
Decrease in boiling temperature in °F:
ΔT(°F) = (3.1 °C × 9/5) + 32
ΔT(°F) = 5.58 °F
Decrease in boiling temperature in °R:
ΔT(°R) = ΔT(°F) + 459.67
ΔT(°R) = 464.58 °R
Learn more about temperature -
brainly.com/question/4735135
#SPJ11
The volume of an object is 55mL in this city is 30g/mL. what is its mass
Answers
Answer:
Mass = 1650g
Explanation:
30g per mL is the density I suppose...
Solve for m!
30g/mL = m/55mL
Multiply by denominator to get numerator!
30* 55 =1650g
m = 1650g
Brainliest Appreciated!
When the volume of a gas is changed from 3. 75 L to 6. 52 L, the temperature will change from 100. 0 K to __ K.
Answers
When the volume of a gas is changed from 3.75 L to 6.52 L, the temperature will change from 100. 0 K to 173.86 K.
How we calculate temperature?Temperature of the given gas will be calculated by rearranging the ideal gas equation as:
V₁/T₁ = V₂/T₂, where
V₁ = initial volume of gas = 3.75L
T₁ = initial temperature of gas = 100K
V₂ = final volume of gas = 6.52L
T₂ = final temperature of gas = to find?
Putting all these values on the above equation and calculate for the value of T₂ as:
T₂ = (6.52 × 100) / 3.75 = 173.86 K
Hence, 173.86 K is the required temperature.
To know more about ideal gas equation, visit the below link:
https://brainly.com/question/25290815
Silver jewelry is a mixture of silver and copper. If a bracelet has a mass of 23.56 g, and it is 80.1% silver, the mass of silver in the bracelet is Group of answer choices
Answers
Answer:
18.87 g
Explanation:
80.1% of 23.56 g = 18.87 g is the mass of the silver
A car travels 350 km in 7 hours. What is the average speed of the car (in km/hr)?
Answers
Answer:
50 km/hr
Explanation:
just divide the distance by the time (350/7)
Answer:
50 km/hr
Explanation:
Distance = 350 km
Time = 7 hours
Average Speed = ?
\(Average\:Speed = \frac{Distance}{Time} \\\\Average\: speed = \frac{350\:km}{7\:hr} \\\\A.V = 50\:km/hr\)
what is the hazardous substance in automobile exhaust that is also present in high concentrations in tobacco smoke?
Answers
The hazardous substance in automobile exhaust that is also present in high concentration in tobacco smoke is acrolein.
Substance that tobacco smoke and the exhaust of automobiles contains a has it is substance called acrolein in very very high concentration.
Inhaling acrolein can give rise to several breathing problems like shortness in breathing and also irritation in lungs.
Acrolein is formed when the fats are overheated apart from automobiles and tobacco a very low amount of acrolein is also found in over used cooking oil.
To reduce acrolein from the automobile exhaust lead based adsorption substances are preferred to be used in automobile exhaust.
To know more about acrolein, Visit,
https://brainly.com/question/6224949
#SPJ4
HELP ME PLEASEEE ILL MARk u the brainliest

Answers
Answer:
3rd or 4th answer is correct
OPTION B is the correct answer

Hey can somebody please give me the answer to this.

Answers
Answer:
it is d or b hope this help
Answer:
A
Explanation:
Question 1 of 10
Which two terms apply to oceanic crust rather than continental crust?
A. Thicker
O B. Lighter in color
0 C. Denser
I D. Younger in age
Answers
The two terms apply to oceanic crust rather than continental crust C. Denser and D. Younger in age
What is the oceanic crust made up of?
Oceanic Crust Oceanic crust, extending 5-10 kilometers (3-6 kilometers) beneath the ocean floor, is mostly composed of different types of basalts. Geologists often refer to the rocks of the oceanic crust as “sima.” Sima stands for silicate and magnesium, the most abundant minerals in oceanic crust.
What is an example of oceanic crust?Oceanic crust is thin (6 km thick) and dense (about 3.3 g/cm), consisting of basalt, gabbro, and peridotite. They include oceanic sediments (e.g. radiolarites, turbidites) and oceanic crust (e.g. basalt, pillow lava).
Learn more about Oceanic crust here https://brainly.com/question/26053779
#SPJ2
which of the following describes a compound? (hint: carbon and oxegen both appear on the periodic table.) A. a piece of pure cotton, containing only carbon atoms B. oxygen gas surrounding a solid piece of carbon C. a substance made of two oxygen atoms for each carbon atom D. carbon and oxygen atoms mixed whithout being bonded together
Answers
Answer:
C. a substance made of two oxygen atoms for each carbon atom
Please answer quickly!! 20 points!!9. Which represents the greatest mass of chlorine?
A) 1 mole of chlorine
B) 1 atom of chlorine
C) 1 gram of chlorine
D) 1 molecule of chlorine
Answers
Answer:
1 mole
Explanation:
if correct you may follow me for more helps
Among the given quantities, the greatest mass of chlorine is represented by one molecule of chlorine Cl₂ that is equal to 71 grams.
What is one molecules?A molecule of an element is formed by the combination of two atoms of that element. One mole of an element is the amount containing 6.022 × 10²³ atoms. This s number is called Avogadro number.
The mass of one mole of an element is called its atomic mass. Cl is 17th element. Atomic mass of Cl = 35.5 g
35.5 g of Cl is called one mole of Cl containing Avogadro number of atoms.
One molecule of Cl is represented as Cl₂ with the mass = 35.5 × 2 = 71 g.
This is the molecular mass of Cl.
One gram of Cl is small amount and mass of one atom of Cl is even smaller. Thus, one molecules of Cl represents the greater mass here. Hence, option D is correct.
Find more on molecular mass:
https://brainly.com/question/3182776
#SPJ2
Do the rocks get older or younger as you go away from the Mid Atlantic Ridge
towards the continents? Why? EXPLAIN.
Answers
Answer:
Rocks get older as we go away from the Mid Atlantic Ridge towards the continents
Explanation:
As the plates move away from each other, new ocean lithosphere is created at the ridge. This also widens the ocean basin and leads to sea floor spreading.
The rock of the ocean floor are symmetrically aligned and they get become older as they move away from the crest of the ridge.
Thus, rocks get older as we go away from the Mid Atlantic Ridge towards the continents
What is the mass of 4.49 x 10º carbon dioxide, CO2, molecules?
Answers
4.49 x 10^0 / 44 g/mol = .1020 mol CO2
1 mol = 6.022 x 10^23 molecules (avogadros number)
so .1020 mol x 6.022 x 10^23 =
6.14 x 10 ^22 molecules CO2
3. Classify each as a physical property or a chemical
property.
a. Aluminum has a silvery color.
b. Gold has a density of 19 g/cm”.
c. Sodium ignites when dropped in water.
d. Water boils at 100°C
e. Silver tarnishes.
f. Mercury is a liquid at room temperature.
Answers
We can classify the following properties as:
a. Aluminum has a silvery color. Physical property. b. Gold has a density of 19 g/cm³. Physical property. c. Sodium ignites when dropped in water. Chemical property. d. Water boils at 100°C. Physical property. e. Silver tarnishes. Chemical property. f. Mercury is a liquid at room temperature. Physical property.The properties of matter can be:
A physical property: a characteristic of a substance that can be observed or measured without changing the identity of the substance. A chemical property: describes the ability of a substance to undergo a specific chemical change.Classify each as a physical property or a chemical property.
a. Aluminum has a silvery color. Physical property. Aluminum doesn't change by observing its color. b. Gold has a density of 19 g/cm³. Physical property. Gold doesn't change by measuring its density. c. Sodium ignites when dropped in water. Chemical property. Sodium reacts with water to form sodium hydroxide and hydrogen. d. Water boils at 100°C. Physical property. Water changes from the liquid to the gaseous state, but it still is water. e. Silver tarnishes. Chemical property. Silver reacts with sulfur-containing compounds in the air to form silver sulfides. f. Mercury is a liquid at room temperature. Physical property. Mercury doesn't change by observing its state of aggregation.We can classify the following properties as:
a. Aluminum has a silvery color. Physical property. b. Gold has a density of 19 g/cm³. Physical property. c. Sodium ignites when dropped in water. Chemical property. d. Water boils at 100°C. Physical property. e. Silver tarnishes. Chemical property. f. Mercury is a liquid at room temperature. Physical property.Learn more: https://brainly.com/question/1935242
A spectrophotometric method for the analysis of iron has a linear calibration curve for standards of 0. 00, 5. 00, 10. 00, 15. 00, and 20. 00 ppm. An iron ore sample with an expected iron content of 40–60% w/w is to be analyzed by this method. An approximately 0. 5 g sample is taken, dissolved in a minimum of concentrated HCl, and diluted to 1 L in a volumetric flask using distilled water. A 5. 00-mL aliquot is removed with a pipet. To what volume (10, 25, 50, 100, 250, 500, or 1000 mL) should it be diluted to minimize the uncertainty in the analysis? Explain
Answers
To calculate the concentration of the iron sample by using a spectrophotometric method, it is necessary to dilute the sample. The volume to which the sample should be diluted is a crucial question in achieving the most accurate result.
The process involves diluting the sample, and the concentration must be calculated to determine the precise result of the dilution. This question can be answered by calculating the uncertainty and identifying the value of the uncertainty. The value with the lowest uncertainty will be the best value to choose. The volume with the lowest uncertainty will be the ideal volume to dilute the 5 ml aliquot of the iron sample to achieve a result with the minimum level of uncertainty.
To determine the optimal volume for dilution, the uncertainty should be calculated.
This can be done by using the equation for propagation of uncertainty, which states that the uncertainty of the result is equal to the square root of the sum of the squares of the uncertainties of the individual components. When calculating the uncertainty of the diluted sample, the uncertainty of the initial sample and the uncertainty of the diluent must be considered. The uncertainty of the initial sample can be calculated using the calibration curve. As the expected iron content is 40-60%, the concentration of the sample is expected to be 8-12 ppm. The uncertainty of the calibration curve is given by the standard deviation of the calibration standards.
The diluent has a negligible uncertainty. The uncertainty of the diluted sample will be lower if a larger volume is used for dilution because the relative contribution of the uncertainty of the initial sample will decrease. However, the uncertainty of the measurement will increase if the sample is diluted too much because the concentration of the analyte will be too low to be detected accurately. A 100 mL volume is a good choice because it balances the need for sufficient dilution to reduce the uncertainty of the initial sample with the need for sufficient concentration to allow for accurate detection of the analyte.
The volume of the sample that should be diluted is 5 ml. The minimum level of uncertainty is obtained at a dilution of 100 ml. When the volume of the diluent is greater than 100 ml, the uncertainty of the measurement increases, and when the volume of the diluent is less than 100 ml, the uncertainty of the measurement also increases. Thus, a 100 ml volume of diluent is the ideal volume to minimize the uncertainty in the analysis of iron.
to know more about spectrophotometric visit:
brainly.com/question/31632843
#SPJ11
A single atom of an element has 11 protons, 11 electrons, and 12 neutrons. Which element is it? a V b Na c Mg d Se
Answers
Answer:
B. Na
Explanation:
To identify an atom, you simply need to look at the number of protons. This atom has 11 protons. On the periodic table, you can see that the element with 11 protons is sodium (Na).
What causes the sea floor to move apart at a sea floor spreading center A density B continental drift C paleomagnetism D convection currents
Answers
Answer: D convection currents
Explanation:
The seafloor spreading is a phenomena that occurs due to liberation of heat from the convection currents generated in the mantle. It makes the earth crust more plastic and less dense. This happens at divergent plate boundaries. As the plates move apart, the less denser material rises. It leads to the formation of mountain and crust cracks.
Which statement about the electron-cloud model is true?
It is the currently accepted atomic model.
It can easily be replaced by existing models.
It specifies the location and momentum of an electron.
It does not explain the formation of emission lines.
DONT WATCH AN AD ITS (A)!
Answers
Explanation:
The statement "It is the currently accepted atomic model" is true. The electron-cloud model, also known as the electron cloud or electron orbital model, is the currently accepted model of the atom. It describes the behavior of electrons in an atom by representing them as existing in regions of high probability called electron clouds or orbitals. This model successfully explains many properties and behaviors of atoms and has been widely accepted by the scientific community.
what is the total contribution of the three si atoms to the sum of oxidation numbers in k-feldspars?
Answers
The sum of oxidation numbers of the three Si atoms in k-feldspars (KAlSi3O8) is +12
What is an oxidation number?The amount of electrons any atom or ion however has gained or lost in comparison to a neutral atom is known as the oxidation number / state of the atom or ion.
k-feldspars, molecular formulae KAlSi3O8
We know that -
Oxidation state of oxygen = -2
Oxidation state of Potassium = +1
Oxidation state of aluminum = +3
8 oxygens, each of them -2, = -16
1 potassium, +1
1 aluminum, +3
1 + 3 - 16 = -12
So the three silicon atoms need to make up +12 for the compound to be neutral.
12 / 3 = +4, so each silicon is +4.
To know more about oxidation number visit :
https://brainly.com/question/6357678
#SPJ4
If 3/5 < x < 67%, which of the following could be the value of x?
Answers
Answer:
2/3, or .66
Explanation:
Call each of your flowers as water employee increase rate of growth
Answers
Explain how atomic interactions determine a material to be transparent and opaque
Answers
Answer:
The materials are opaque or crystalline from a client to the orientation and type of union between their atoms, forming two types of structures.
These two structures can be crystalline or amorphous.
In the case of being crystalline, these unions do not allow light to pass through the medium of the object or body of said compound, making it totally refract and giving the appearance of OPAQUE.
On the other hand, in those compounds that we call amorphous, the atoms are located in a different way that makes light pass through them, without absorbing or identifying any light beam, so they look transparent.
Explanation:
Example: A glass cup has an amorphous structure, while a porcelain or porcelain plate has a crystalline structure.
The part of the water cycle where water from the ocean is changed into vapor is
driven by energy from the ?
A) lunar tides
B) earth's gravity
C) magnitisim
D) sun's heat
Answers
Answer:
D) Sun's heat
Explanation:
The water cycle is driven primarily by the energy from the sun. This solar energy drives the cycle by evaporating water from the oceans, lakes and rivers.
Answer:
D) sun's heat
Explanation:
The sun's heat provides enough energy to water molecules at the surface so they move fast enough to escape from the water. The water does not need to boil.
What’s the answer?????
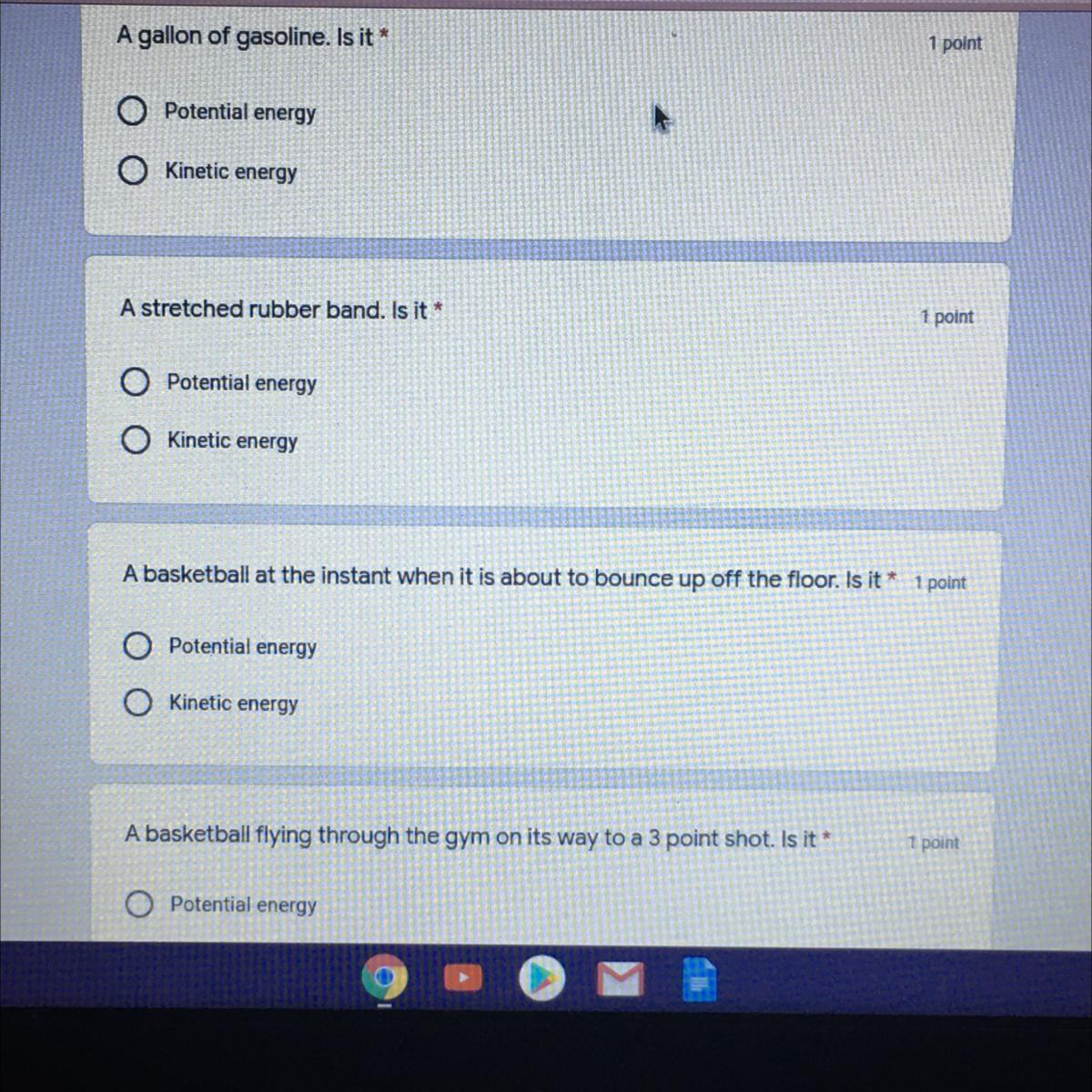
Answers
potential energy
kinetic energy
kinetic energy
idk last sorry =(
What is the temperature of a 6.1 mole sample of He gas at 18.5 bar and volume of 115L? (Round answer to the hundredths place)
Answers
Answer:
4202.1 K
Explanation:
From;
PV=nRT
P= pressure of the gas = 18.5 bar
V = volume of the gas = 115L
n= number of moles of the gas = 6.1 mole
T= temperature of the gas = ?
R = gas constant = 0.083 L⋅bar⋅K−1⋅mol−1
T = PV/nR
T = 18.5 * 115/6.1 * 0.083
T = 2127.5/0.5063
T= 4202.1 K
A 18 g sample of metal is heated to 96°C and placed in a calorimeter containing 21 g of water at an initial temperature of 24°C. After the metal cools in the water bath, the final temperature inside the calorimeter is 26°C. What is the (positive) specific heat of the metal rounded to the HUNDREDTHS place? (hint: final temperatures of metal and water are the same). Do NOT include units. *
Answers
Answer: 140
Explanation:
Given the following :
Mass of metal = 18g = 0.018kg
Mass of water (calorimeter) = 21g = 0.021kg
Initial temperature of metal = 273 + 96 = 369k
Initial temperature of water = 273 + 24 = 297k
Final temperature inside calorimeter = 273 + 26 = 299k
Temperature change of metal = 299 - 369= -70k
Temperature change of water = 299 - 297 = 2k
H = mc ΔT
m = mass
c = specific heat capacity
ΔT = change in temperature
Heat lost by metal = heat gained by water
mc ΔT = mc ΔT
Specific heat capacity of water = 4200
18 * C * ( 299 - 369 ) = 21 * 4.2 * ( 299 - 297)
0.018 × C × -70 = 0.021 × 4.2 × 2
- 1.26 × C = 0.1764
C = 176.4/1.26
C = 140
2. If a gas at 500 mL has a temperature of 45°C, then what is the new volume when the temperature is increased to 65°C? Show your work.
Answers
Answer:
V2= 531.4 ml
Explanation:
T1= 273+45 °C= 318 Kelvin
T2= 273+65 °C= 338 Kelvin
V1= 500ml *( 0.001L/1ml)= 0.5 Liters
V2= \(\frac{V1 * T2}{T1}\)= \(\frac{0.5 L * 338K}{318K}\) = 0.5314465409 Liters* (1 ml/0.001 L)= 531.4 ml
im giving out the game the last of us part 2 who wants it
Answers
Answer:
YEESSS
Explanation:
Because that seems like the only logical answer.
Answer:
YES
Explanation:
YES
How much time will it take a car traveling at 65 miles per hour to travel 1600 mile?
Answers
Answer:
24.61 hours to travel 1600 miles
Explanation:
What we want is how many hours it takes to travel. So we are looking for the hours, whenever you see something like 1600 miles and 65 miles imagine it as a fraction. 1600/65
Then make it a decimal which would be 24.61
You got you're answer :D
Answer:
24.61 hours to travel 1600 miles
Explanation: