Answers
Answer:
16,,24Mg 17,,a24.1 18a mass number of the most abundant isotope
Explanation:
atomic number of Mg is 12 ,therefore its mass number should be the value that is very close to 24.
24.1 is the value of thee most abundant isotope.
Related Questions
What is the function of the esophagus?
Answers
The esophagus functions primarily as a transport tube that directs the progression of food and fluids from the mouth to the stomach.
A graduated cylinder contains 4.50 mL of water. After a piece of zinc is dropped into the cylinder, the water level rises to 9.24 mL. What is the volume of the piece of zinc?
Answers
Answer: Well, the volume of the copper is (63.4 - 40.0) * mL = 23.4 * mL
Explanation:
Do you agree? The copper displaces the given volume of water.
Now ρ Cu = 8.90 ⋅ g ⋅ c m 3 OR ρ Cu = 8.90 ⋅ g ⋅ m L − 1, i.e. 1 ⋅ m L ≡ 1 ⋅ c m 3
But by definition, ρ density = mass volume
And thus mass = ρ × volume = 8.90 ⋅ g ⋅ m L − 1 × 23.4 ⋅ m L
= 208.3 * G
3. If an element has 47 protons and 54 neutrons what is the element and what is its atomic
mass?
4. If one atom has 47 neutrons and a mass of 87 and another one has 41 protons and a
mass of 87, are they isotopes of each other?
5. Draw the electron dot diagram for the element Phosphorous.
Answers
4.Nb(Niobium) the atomic mass is 92.906
5.

I need help with this i need help by sb

Answers
Answer:
30N to the left.
Explanation:
There is no net vertical force as the 45N force upwards and the 45N force downwards cancel each other out.
Horizontally, the resultant force is the 30N to the left as the 45N force to the left is in opposite direction to the 15N force to the right, so they are subtracted:
45N - 15N = 30N
Hope this helps!
Prove how the Law Conservation of Mass and Enery apply to Cellular Respiration
Answers
Answer:
mass and energy is reused when those things happen
What happened to the substances in the test tube during Step 2?
A. The heat started a chemical reaction between the iron and sulfur, making the compound
iron sulfide.
B. The heat started a chemical reaction between the iron and sulfur, making the element iron
sulfide.
C.
D.
The heat started a physical reaction between the iron and sulfur, making the solution iron
sulfide.
The heat started a physical reaction between the iron and sulfur, making the mixture iron
sulfide.

Answers
Answer:
A the heat started a chemical reactions
The heat started a chemical reaction between the iron and sulfur, making the compound iron sulfide. So, the correct option is (A).
What is Chemical Reaction?
A chemical reaction is defined as the process that leads to a chemical change from one set of chemical substances to another.
In this process, a substance or substance undergoes a chemical change to produce a new substance or substances, with whole new properties, in a process known as chemical reactions. The nature and identity of the products is completely different from that of the reactants.
For the information above, the chemical reaction caused by the heat, the iron and the suphur mixed with each other are heated on a burner forming a yellow color, which turns into iron sulphide, a black colored compound having totally different properties.
Thus, the heat started a chemical reaction between the iron and sulfur, making the compound iron sulfide. So, the correct option is (A).
Learn more about Chemical Reactions, here:
https://brainly.com/question/29039149
#SPJ2
As part of an investigation of the population of foxes on Sunday Gill island a scientist graphed the number of foxes presented on the island over a Spam of 15 years as shown below the study began with the earlier 0 and run until the start of year 15 According to the graph during the witch year the event reduced the carrying capacity of the area
Answers
The carrying capacity of the area was reduced in the year 10 according to the graph that shows the number of foxes on the island over a span of 15 years.
The graph shows a population of foxes over a span of 15 years. The y-axis represents the number of foxes on the island, while the x-axis represents time. The study began with the earlier 0 and ran until the start of year 15. According to the graph, the carrying capacity of the area was reduced in the year 10.
In the graph, it is shown that the population of foxes on Sunday Gill island had a significant increase from year 0 to year 3. After year 3, the fox population started to decrease and then remained fairly constant until year 10. After year 10, the population of foxes on the island started to decline more rapidly until the end of the study in year 15
This decline in the population of foxes on the island is most likely due to the reduction in carrying capacity of the area. Carrying capacity refers to the maximum number of individuals that an environment can sustain. When the carrying capacity of an environment is reached, it means that the environment can no longer provide the necessary resources to sustain the population.
There are various factors that can cause a reduction in carrying capacity, such as environmental degradation, competition for resources, or a natural disaster. In this case, it is not clear what caused the reduction in carrying capacity in year 10, but it is likely that it was due to some environmental factor that impacted the availability of resources for the fox population.
For more such information on: graph
https://brainly.com/question/31305548
#SPJ8
how many elements are present in the compound
Answers
go play mine craft use code wild ............................
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
List two characteristics of a calorimeter that are necessary for successful heat measurement.
Answers
Two characteristics of a calorimeter that are necessary for successful heat measurement are good thermal insulation to minimize heat loss and a temperature measurement device to accurately measure temperature changes.
How does a calorimeter work?A calorimeter works by measuring the heat exchanged between two substances in a chemical or physical process. The substances are placed in the calorimeter and allowed to reach thermal equilibrium, and the temperature change of the system is measured to calculate the amount of heat transferred.
What is the difference between a bomb calorimeter and a coffee cup calorimeter?A bomb calorimeter is designed for measuring the heat of combustion of a substance in a sealed container under constant volume conditions, while a coffee cup calorimeter is a simpler design used for measuring the heat of reaction of a substance in an open container under constant pressure conditions. The bomb calorimeter is typically more accurate but also more complex and expensive to use.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
which term describes the repeated arrangement of the same molecule; total number of c h bonds in propanone is
Answers
The term (D) extended structure describes the repeated arrangement of the same molecule.
The extended structure is a type of structure that may be described as one in which the subunits are organized in a pattern that is repeated and occur in a ratio that is constant. Diamond and sodium chloride are two examples of extended structures. Extended structures have well-separated hydrophilic and hydrophobic layers made of propagating chains along the calixarene cavity axis. Therefore, the word "extended structure" is the one that is used to describe the repeating arrangement of the same molecules. So, choice D is the right one.
The correct question is as:
Which term describes the repeated arrangement of the molecule?
A. Bonds
B. Atoms
C. Molecular Model
D. Extended Structure
You can also learn about extended structure from the following question:
https://brainly.com/question/12077155
#SPJ4
in two or more sentences, describe two types of data that can be obtained from descriptive statistics that you learned about in your project. in your description explain how the data is calculated
Answers
Two types of data that can be obtained from descriptive statistics include frequency distribution and variability in the data set. These data are calculated as the mean of standard deviation in the case of variation.
What is the relative importance of the science field of descriptive statistics?The relative importance of the science field of descriptive statistics is based on the obtention of statistically significant measurements in order to validate a hypothesis.
Therefore, with this data, we can see that the relative importance of the science field of descriptive statistics is based on testing working hypotheses.
Learn more about descriptive statistics here:
https://brainly.com/question/6990681
#SPJ1
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Given 5.86 grams of NaN3, how many grams of Na are produced?
Answers
Answer:
what no entiendo nada jahag6
When 5.86 grams of NaN3, then 22.99 gram of Na are produced by using mole formula.
What is gram ?A thousandth of a kilo-gram in terms of mass in the metric system.
What is mole?A mole is a unit of measurement for atom amount. A mole of Iron atoms weighs heavier than just a mole of Carbon molecules because it is measured by the number of atoms present instead of by weight.
The reaction can be written as:
\(2 Na N_{3}\) → 2Na + \(3N_{3}\).
It can be seen that 2 mole of sodium azide give 3 mole of nitrogen molecule.
Calculate the moles of Na.
Moles of Na = 1 mol sodium azide × 2 mol of Na / 2 mol sodium azide = 1 mole of Na.
Calculate the mass of Na.
Mass of Na = 1 mol Na × 22.99 g Na / 1 mol Na = 22.99 gNa.
Therefore, the mass of Na will be 22.99 gram
To know more about mole.
https://brainly.com/question/26416088
#SPJ3
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
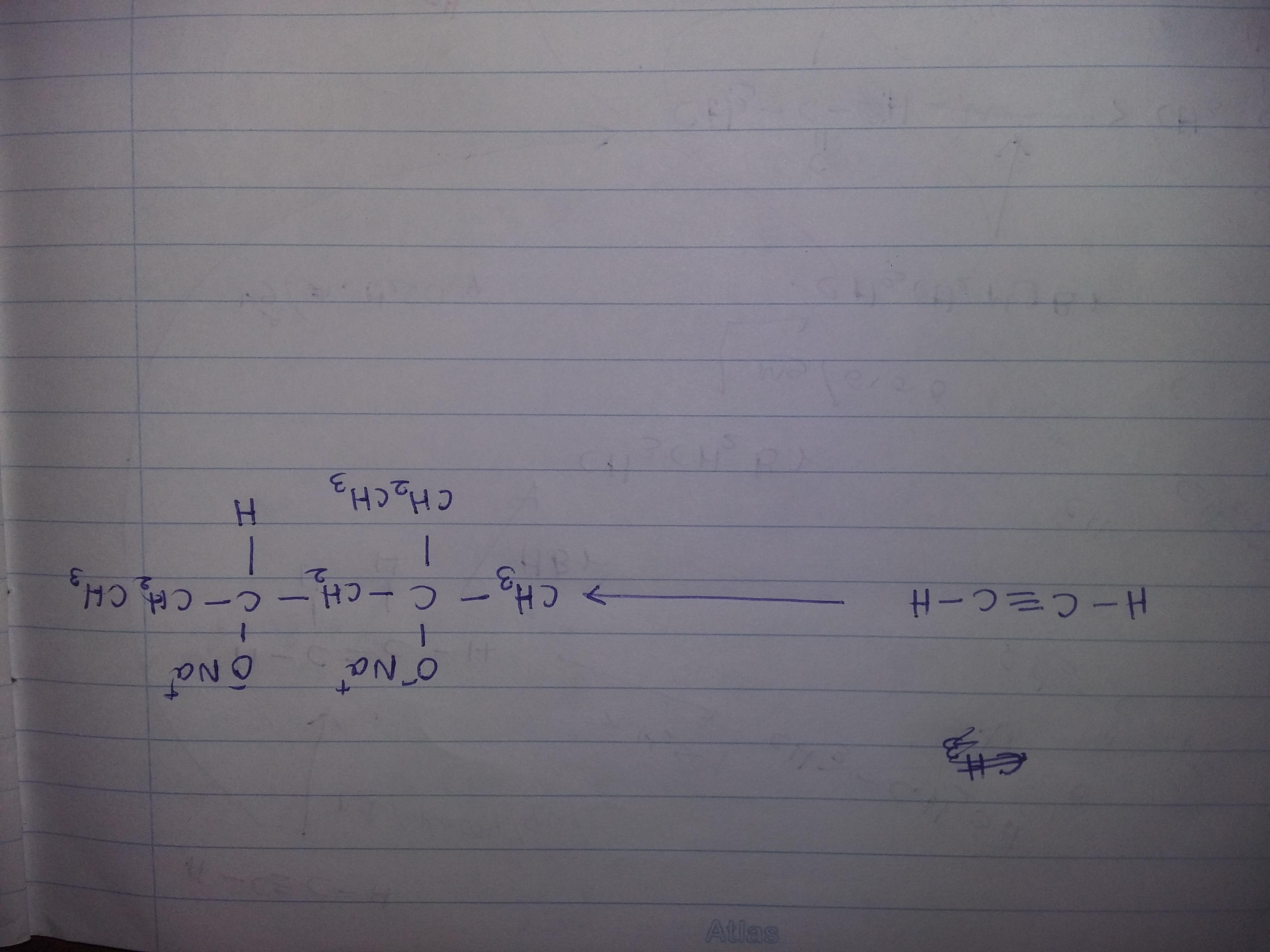
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
What is the mass of 1.78 moles of O2
Answers
Answer:
56.96 grams
Explanation:
To find the mass of 1.78 moles of O2, we need to use the molar mass of O2, which is the mass of one mole of O2.
The chemical formula for O2 is O-O or simply O2. The molar mass of O2 is the sum of the atomic masses of two oxygen atoms, which can be found on the periodic table.
The atomic mass of oxygen (O) is approximately 16.00 g/mol. So the molar mass of O2 is:
Molar mass of O2 = 2 x atomic mass of O
= 2 x 16.00 g/mol
= 32.00 g/mol
Therefore, the mass of 1.78 moles of O2 is:
Mass = number of moles × molar mass
= 1.78 mol × 32.00 g/mol
= 56.96 g
So the mass of 1.78 moles of O2 is 56.96 grams.
6. A box measures 11.25 inches in length, 8.1 inches in width and 6.85 inches in height. What is the
volume of the box?
Answers
Answer:
I'd say 624.2^3 inches.
Explanation:
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
8. A 25.0 mL sample of an H2SO4 solution is titrated with a 0.186 M NaOH solution. The equivalence point is reached with 12.9 mL of base. The concentration of H2SO4 is ________ M. (Hint: write a balanced chemical equation first!)
Answers
Answer:
0.0480 M
Explanation:
The reaction is ...
H₂SO₄ + 2NaOH ⇒ Na₂SO₄ +2H₂O
That is, 2 moles of NaOH react with each mole of H₂SO₄. Then the molarity of the H₂SO₄ is ...
moles/liter = (0.186 M/2)(12.9 mL)/(25.0 mL) ≈ 0.0480 M
NaOH + HCl → NaCl + H2O
What mass of water forms when 2.75 × 10–4 mol NaOH reacts completely?
Answers
Answer:
0.00496 g H2O
Explanation:
Answer:
0.00496
Explanation:
trust
10. Explain the relationship between the group number and the valence electrons
of MAIN GROUP elements (Groups 1, 2, 13-18). *
Answers
Valence electrons are electrons that are in the outermost orbitals of an atom. In the periodic table the elements are divided into groups. All elements of the first main group have one valence electron. All elements of the second main group have two valence electrons. And so on:
group 1 - 1 valence electrongroup 2 - 2 valence electronsgroup 13- 3 valence electronsgroup 14 - 4 valence electronsgroup 15 - 5 valence electronsgroup 16 - 6 valence electronsgroup 17 - 7 valence electronsgroup 18 - 8 valence electronsMean, median and mode are best described as which?
A. Qualitative and quantitative data
B. Measures of central tendency
C. Discrete and continuous data
D. Histogram, scatterplot and circle
Answers
Answer:
B. Measures of central tendency
Explanation:
Mean, median and mode are best described as measures of central tendency of a given data set.
Mean is the average of the samples given
Mode is the data point with the most frequent occurrence
Median is the data point that lies in the middle
All these parameters tells us how far a data point is from the middle or how close they are.Carry out the following conversions. Report your answers to the correct number of significant
figures.
(a) 75 cm to ft
ft
(b) 0.668 kg to lb
Ib
(e) 0.549 uL to qt
* 10-qt
(d) 9,991 mm to km
9.991 x 10 (select)
km
(e) 313 nm to mm
3.13 x 10 (select)
mm
(1) 7 ft to cm
* 102 cm
Answers
We want to perform some changes of units, such that the number of significant figures (the ones different than zero) does not change.
a) 75 cm to ft
We know that:
1ft = 30.48cm
1 = (1ft)/(30.48cm)
Now, remember that if we multiply a quantity by one we do not affect the quantity, so we can write:
75cm = 75cm*(1ft)/(30.48cm) = 2.5 ft
b) 0.668 kg to lb
we know that:
1kg = 2.20462 lb
then:
1 = (2.20462 lb)/(1kg)
0.668 kg = 0.668 kg*(2.20462 lb)/(1kg) = 1.47 lb
c) 0.549 uL to qt
We know that:
1 uL = 3.437*10^-9 qt
1 = (3.437*10^-9 qt)/(1 uL)
Then:
0.549 uL = 0.549 uL*(3.437*10^-9 qt)/(1 uL) = 1.91*10^-9 qt
d) 9.991 mm to km
we know that:
1mm = 1*10^-6 km
then:
1 = (1*10^-6 km)/(1mm)
So we can rewrite:
9.991 mm = 9.991 mm*(1*10^-6 km)/(1mm) = 9.991*10^-6 km
e) 313 nm to mm
We have:
1nm = 1*10^-6 mm
1 = ( 1*10^-6 mm)/(1 nm)
Then:
313 nm*( 1*10^-6 mm)/(1 nm) = 3.13*10^-4 mm
If you want to learn more, you can read:
https://brainly.com/question/24606249
A blacksmith is in the process of making iron horse shoes. Each shoe, mass of 255.15g, is heated to a bright red (816c), hammered into shape, and then dropped into water to cool. If the bucket contains 4L of water (4000g) initially at room temperature (23c) what will the final temperature of the iron be? Specific heat of cast iron: 0.461 J/gC. Specific heat of water: 4.18 J/gC
Answers
Answer:
Explain
The Akautski is now assembled
The final temperature of the system can be calculated using calorimetric equation. The heat gained by water is equal to the heat lost by iron. Hence, the final temperature is 28 °C.
What is calorimetry ?Calorimetry is an analytical tool used to determine the heat energy absorbed or released by a system. The calorimetric equation connecting the heat energy q with mass m, specific heat capacity c and temperature difference ΔT is given as:
q = m c ΔT
Here, the heat lost by iron = heat gained by water
m of iron = 255.15 g
initial temperature = 816 °C
c = 0.461 J/°C g.
Mass of water = 4000 g
c = 4.18 J/°C g
initial temperature = 23 °C
Let T be the final temperature of the system.
then,
0.461 J/°C g × 255.15 g × (816 - T) = 4.18J/°C g × 4000 g × (T - 23)
117.62 J(816 - T) = 16720 (T - 23)
T = 28°C.
Therefore, the final temperature of the system will be 28°C.
Find more on calorimetry:
https://brainly.com/question/11477213
#SPJ7
someone help with this will mark brainliest

Answers
Answer:
The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum, whereas an absorption spectrum has dark-coloured lines in the spectrum.l
a) During the workup of the reaction, an aqueous solution of sodium bicarbonate was added to the cooled reaction mixture. Why was this done
Answers
Answer:
The purpose of adding an aqueous solution of sodium bicarbonate is either to extract a certain compound or to remove/neutralize acidic compounds present in the reaction mixture.
Explanation:
Upon adding sodium bicarbonate, carbon dioxide is released (gaseous state at room temperature), which helps build up pressure that is able to push out the unwanted gas/liquid.
2. A company makes mixtures of acetic acid and water such that the acetic acid is 15% of the total mass (weight) of the mixture. Let A be an unspecified number of grams of acetic acid, which can vary and let W be the corresponding number of grams of water in this type of mixture.
An equation that relates A and W is A = (3/17) W.
Answers
The equation that relates A and W, considering the desired 15% acetic acid concentration, is 3W = 2.55M.
The equation A = (3/17)W represents the relationship between the mass of acetic acid (A) and the mass of water (W) in the mixture. It states that the mass of acetic acid is equal to three seventeenths (3/17) of the mass of water.
Since the company wants the acetic acid to be 15% of the total mass of the mixture, we can set up another equation to represent this requirement. Let M be the total mass of the mixture. The mass of acetic acid (A) is 15% of the total mass, so we have A = 0.15M.
Now we can substitute A in terms of W from the first equation into the second equation: (3/17)W = 0.15M. We can simplify this equation by multiplying both sides by 17 to get 3W = 2.55M.
This equation allows the company to calculate the mass of water (W) required for a given mass of acetic acid (A) to maintain the desired concentration in the mixture.
For such more questions on concentration
https://brainly.com/question/26175405
#SPJ8
When an iron metal is heated to a temperature of 200 degrees C and placed in a water at 25 degrees C The heat energy be transferred from*
Answers
Answer:
Heat energy is transferred from the iron metal to the water.
Explanation:
Heat energy is transferred from the iron metal to the water. The process in which this occurs is referred to as heat transfer. The type of heat transfer that occurs here is referred to as conduction. Conduction is a type of heat transfer involving the transfer of heat energy from a substance with higher temperature to a substance with lower temperature by direct contact.
In the case of the question, the iron metal has a higher temperature of 200 degrees and is brought in contact with another substance (water) with a lower temperature of 25 degrees.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
help pls
plspspslslslsl

Answers
Answer:
see below
Explanation:
K.E. = 1/2 * m * v^2
= 1/2 * 2 * 5^2 j
