If 100 grams of O2 are reacted, how many grams of P4 will also be reacted?
Answers
If 100 grams of Oxygen are reacted, 77.5 grams of P4 will also be reacted.
Calculating the number of moles in 100g of oxygen gas.
Mass of oxygen = 100 g
Molar mass of oxygen = 32.0 g/mol
Thus, moles of oxygen are -
Moles of oxygen = (Mass of oxygen/ Molar mass of oxygen)
Moles of oxygen = (100/32.0)
Moles of oxygen = 3.125 moles.
Here, the 5 moles of Oxygen need 1 mole of P4.
Moles of Phosphorous = 3.125/5 = 0.625 moles.
Therefore, from moles of P4 and molar mass of P4 = (124.0 g/mol)
Mass of Phosphorous = 0.625 * 124.0 = 77.5g
Hence, 77.5 grams of P4 will react with 100 grams of Oxygen.
To learn more about Moles and Mass
https://brainly.com/question/13860160
#SPJ4
Related Questions
When determining whether a chemical change has taken place, you observe and look for several indicators. Which would be considered an indication that a chemical reaction has taken place?
a Heat is given off.
b Solid melts.
c An object has changed shape.
d Substance dissolved.
Answers
A chemical change occurs when there is a bond breaking or bond making. A phase change, change in phase and or change in shape does not make a chemical change. A chemical change can be indicated by the heat given off by a reaction or change. Thus, option a is correct.
What is chemical change?There are broadly two types of change occurs for a substance namely chemical change or physical change. A physical change is a change in state or phase involves no formation products or breaking of bonds. For example melting of a solid to liquid and boiling of liquid to gas etc.
In a chemical change there makes a bond breaking or making to form a new product. All chemical reactions such as combination reactions, decomposition, displacements etc. are chemical changes.
Every chemical change is associated with an enthalpy of heat energy that is absorbed or released by the system. Thus heat given off is an indication of a chemical change. Hence, option A is correct.
To find more about chemical change, refer the link below:
https://brainly.com/question/8159283
#SPJ1
How are states of matter different at an atomic scale?
Answers
Answer:
The bonds that exist between their atoms and molecules differ on an atomic scale.
Explanation:
The three basic states of matter are solid, liquid, and gas. The major difference between them is the strength of the bonds that hold their molecules together. This affects the way that the molecules interact with each other.
The intermolecular forces binding solids together are very strong. This makes solids rigid and not able to move about freely, but rather, only able to vibrate about a mean position.
The intermolecular forces binding liquid atoms together are a lot weaker than that of solids. This makes liquids able to flow.
The intermolecular forces binding gas molecules together are the weakest. As a result, the gases can move about freely and occupy no definite volume.
Using examples, explain which electrochemistry technology you think is the most cost efficient.
Answers
Among various electrochemistry technologies, lithium-ion batteries are considered the most cost-efficient due to their widespread use, decreasing prices, and high energy density.
Lithium-ion batteries have emerged as the dominant technology for energy storage in portable electronics, electric vehicles, and renewable energy systems. They offer a combination of high energy density, long cycle life, and relatively low self-discharge rates compared to other electrochemical technologies. These factors make them highly cost-efficient in a variety of applications.
One example of the cost efficiency of lithium-ion batteries can be seen in the electric vehicle (EV) market. Over the years, advancements in lithium-ion battery technology and increased production scale have led to significant cost reductions. This has resulted in a decline in the prices of EVs, making them more accessible to consumers. The cost efficiency of lithium-ion batteries has also been demonstrated in the renewable energy sector. Energy storage systems based on lithium-ion batteries allow for efficient integration of intermittent renewable energy sources, such as solar and wind power, into the grid. This helps stabilize the grid and reduce reliance on fossil fuels.
Furthermore, the high energy density of lithium-ion batteries enables compact and lightweight designs, making them suitable for portable electronics like smartphones and laptops. This not only enhances user convenience but also contributes to cost efficiency by reducing material and transportation costs. Additionally, the long cycle life of lithium-ion batteries ensures durability and longevity, further enhancing their cost efficiency as they require fewer replacements over their lifespan.
Learn more about lithium-ion batteries here:
https://brainly.com/question/13651147
#SPJ11
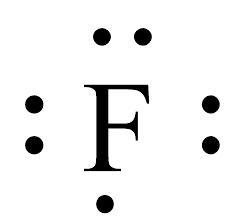
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
i need help please with this chemistry work

Answers
Answer:
question 1: 3
question 2: the number of Valence electrons in the atom
hope it helps
Whoever gets right I will give you Brainly

Answers
Answer:
B
Explanation:
Which worker participates in the primary sector of an economy?
O A. A factory worker
O B. A lawyer
O C. An accountant
D. A farmer
Answers
The answer is A. factory worker
the excess gibbs energy for the chloroform(1)/ethanol(2) system at 55°c is well represented by the margules equation the vapor pressures of chloroform and ethanol at 55°c are psat1
Answers
The excess Gibbs energy for the chloroform(1)/ethanol(2) system at 55°C can be accurately described by the Margules equation. This equation helps us understand the non-ideal behavior of the system by considering the interactions between the different components.
To determine the excess Gibbs energy, we need to know the vapor pressures of chloroform and ethanol at 55°C, which are denoted as psat1 and psat2, respectively. Unfortunately, the question seems to be cut off after mentioning psat1.
Without the complete information about psat1 and psat2, we cannot calculate the excess Gibbs energy or provide further insights into the system. If you have the complete data, please provide it so that we can proceed with the calculations.
In general, excess Gibbs energy quantifies the departure from ideality in a mixture and reflects the energy required to create or destroy intermolecular interactions between the components. It is an important concept in thermodynamics that helps us understand the behavior of mixtures and their phase equilibria.
Learn more about Gibbs energy on-
https://brainly.com/question/9179942
#SPJ11
1. How many miles are in 1,000,000 centimeters
I already know the direct answer I just need help filling in the boxes in the picture above

Answers
What process transfers water from the atmosphere to the hydrosphere
A evaporation
B runoff
C precipitation
D currents
Answers
. a group of students are studying rocks in their science class. they are trying to identify a rock that
has black and white foliated (wavy) stripes.
student a says the rock likely formed when black and white sediments collected together over time and
settled into layers. these layers later cemented together into a sedimentary rock.
student b says the rock is likely formed underground and slowly grew crystals, which is common for
igneous rocks
student c says the rock is likely an igneous rock, such as granite, because it is made of more than one
color.
student d says the rock probably transformed over time. heat and pressure deformed the layers and
turned them into foliated lines often found in metamorphic rocks.
which student do you believe has the most accurate explanation?
a. student a
b. student b
c. student c
d. student d
Answers
Because the rock has multiple colors, student c claims that it is most likely an igneous rock like granite.
Which science focuses on the analysis of rocks and stones?The study of rocks, including igneous, metamorphic, and sedimentary ones, as well as the processes that create and alter them is known as petrology. The study of the chemistry, crystal structure, and physical characteristics of the minerals that make up rocks is known as mineralogy.
What procedure is used to identify rocks?Crystal shape, color, hardness, cleavage, and specific gravity are among the most prevalent physical characteristics. Examining the crystal form of a mineral is one of the finest ways to identify it (external shape). A crystal is described as a homogeneous material with a lattice-defined three-dimensional internal organization.
to know more about rocks here:
brainly.com/question/19930528
#SPJ4
If 8.05 moles of an ideal gas has a pressure of 2.06 atm and a volume of 27.83 L, what is the temperature of the sample
Answers
The temperature of the sample is approximately 85.641 K.
To solve this problem, we can use the ideal gas law equation:
PV = nRT.
Given:
n (number of moles) = 8.05 moles
P (pressure) = 2.06 atm
V (volume) = 27.83 L
We need to find the temperature (T).
R is the ideal gas constant, which is a known value. It is usually given as 0.0821 L·atm/(mol·K).
Let's plug in the values into the equation and solve for T:
PV = nRT
(2.06 atm)(27.83 L) = (8.05 mol)(0.0821 L·atm/(mol·K))(T)
Simplifying the equation:
56.84498 = 0.6647055T
Divide both sides by 0.6647055:
T = 56.84498 / 0.6647055
T ≈ 85.641 K
Therefore, the temperature of the sample is approximately 85.641 K.
Learn more about temperature in the link:
https://brainly.com/question/27944554
#SPJ11
How do I balance this?

Answers
C12H22O11 (s) + 12O2 (g) ⇒ 12CO2 (g) + 11H2O (g)
NaHCO3+HCI--->NaCI+H2O+CO2
Percent yield:93.4%
how would the percent yield be affected if some sodium hydrogen carbonate is left unreacted? explain
Answers
Answer:
Explanation:
percent yield is ratio of actual yield or experimental yield divided by theoretical yield multiplied by 100 .
percent yield of 93.4 % means , the actual yield is 93.4 % what was expected from the reaction on the basis of given chemical reaction .
If in the experimental process , some sodium hydrogen carbonate is left unreacted due to absence of reactant HCl which is also required to obtain product , the percent yield will be increased if the required HCl is also provided .
Hence the percent yield will be increased if required HCl is made available .
1. How did Moseley offering a different explanation than Mendeleev on the organization of elements advance science?
A. It helped prove that there are no undiscovered elements.
B. It established atomic numbers as the basis for the periodic table
C. It helped uncover previously undiscovered elements.
D. It established atomic mass as the basis for the periodic table.
2. Why is empirical knowledge important?
A. It provides science with facts.
B. It lets scientists conduct investigations
C. It turns experiences into facts.
D. It helps scientists make observations
Answers
Moseley offering a different explanation than Mendeleev on the organization of elements advance science by option B. It established atomic numbers as the basis for the periodic table
The empirical knowledge is important by option D. It helps scientists make observations.
What is the explanation about?Moseley's contribution to the organization of elements advanced science by establishing atomic numbers as the basis for the periodic table (Option B). This helped to improve the accuracy of the periodic table, making it possible to predict the properties of new elements based on their atomic number.
Therefore, Empirical knowledge is important because it provides science with facts (Option A). Empirical knowledge is based on observations and experiences, which can be tested and verified through experiments. This helps to build a solid foundation for scientific understanding, as empirical knowledge provides a basis for scientific theories and models.
Learn more about Mendeleev from
https://brainly.com/question/11286917
#SPJ1
t/f do not use oil-based products (vaseline, body lotions) because they destroy latex
Answers
True. Do not use oil-based products such as Vaseline and body lotions because they destroy latex. Latex is a natural rubber, and when it comes into contact with oil-based products, it reacts chemically.
This reaction causes latex to degrade and lose its elasticity, making it prone to breakage. Therefore, it is important to avoid oil-based products when using latex products, such as condoms, gloves, and other medical supplies. Instead, use water-based products that are safe to use with latex. Water-based products are gentle on the skin and do not react chemically with latex, making them ideal for use with latex products.
learn more about chemically
https://brainly.com/question/12145141
#SPJ11
chemistry A Gases unit test
Answers
Answer:
gas is composed to particles and has a mass.
underground rock suddenly breaks and there is rapid motion along a fault
Answers
Answer: Earthquakes are usually caused when underground rock suddenly breaks and there is rapid motion along a fault. This sudden release of energy causes the seismic waves that make the ground shake.
Answer: This would cause an earthquake
Explanation:
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
Convert solar day into mintues
Answers
Answer:
There are 24*60 minutes in a day (ignoring the imperfections of the natural world, the Earth and Sun). So there are 24*60 valid 24 hour times (excluding seconds) on a digital clock.
A measure of time representing the interval between consecutive passages of the Sun across the meridian, averaged over one year. 1 day = 24 hr (86,400 sec) and 60 minutes.
hope it helps ya mate! :-P
Silver is produced as a byproduct of gold
production. Gold and silver are part of
Group 11 on the periodic table. What does
this indicate about silver?
Answers
Answer:
Element Properties
atomic number 47
atomic weight 107.868
melting point 960.8 °C (1,861.4 °F)
boiling point 2,212 °C (4,014 °F)
specific gravity 10.5 (20 °C [68 °F])
oxidation states +1, +2, +3
electron configuration [Kr]4d105s1
hope that helps
A sample of gas occupies 3.00 L with 5.00 moles present. What would
happen to the volume if the number of moles is increased to 8.0?
Answers
3/5 times 5/3x = 8*3/5. X=24/5 simplified would be x= 4.8 L.
Explain briefly why ammonia and oxygen molecules participate readily in coordinate bonding. Give an example for the formation of an ion by ammonia by this means. Show by means of electronic diagram the formation of a coordinate linkage between phosphorus tri chloride and oxygen?
Answers
Explanation:
When one atom supplies both electrons in forming a two-electron bond, it is known as a dative covalent or coordinate bond. ... In this case, the nitrogen atom has a lone pair of electrons that coordinates to the hydrogen cation, which has zero electrons, forming a covalent bond.
A cake is made by mixing ingredients and placing the material in an oven for baking. What type of reaction is involved?.
Answers
If Tins’s mass number is 119 and it’s atomic number is 50, how many neutrons are in the nucleus of an atom of tin?
Answers
Answer:69
Explanation:cause ima boss
Carbon disulfide is a linear molecule with two double bonds. According to valence bond theory, which orbitals on carbon are used for pi bonding?
Answers
According to valence bond theory, 2s and 2p orbital of carbon atom will be formed pi bonding.
One of it's two fundamental theories created to use quantum mechanics to describe chemical bonding would be the valence bond theory, and molecular orbital theory.
Eight valence electrons should be used to generate two double bonds in the CS2 molecule. As a result, it uses up eight of the 16 valence electrons. These valence electrons are located in the carbon atom's 2s and 2p orbitals, where they join to create a double bond.
Therefore, according to valence bond theory, 2s and 2p orbital of carbon atom will be formed pi bonding.
To know more about valence bond theory
https://brainly.com/question/23129240
#SPJ4
*Absolute zero is the temperature when:
Answers
Answer:
It is the temperature at which water is frozen or is pure ice
"Absolutely zero" temperature is the coldest temperature possible. It's so cold that everything stops moving and has no energy left. Scientists use a special scale called Kelvin to measure temperature, and absolute zero is at 0 Kelvin or -273.15 degrees Celsius (-459.67 degrees Fahrenheit). We can't actually reach absolute zero in real life, but scientists have come very close in laboratories using special cooling methods.
Correct the following misconceptions: a) when a solid melts, the particles which make up the solid turn into solid. B) when a liquid boils, the particles which make up the liquid turn into gas. C) When a gas condenses, the particles which make up the gas become liquid
Answers
Answer:
a b c
Explanation:
when a solid melts the particles spread put
when a liquid boils again the particles will spread out
when a gas condenses the particles come closer together
How is a comet different from a star?
Answers
Explanation:
Bottom line: Most asteroids are rocky bodies that lie within the asteroid belt while comets are dirty snowballs from the Oort Cloud, with some objects acting like a hybrid of these two types.
what m/z value would you predict for the most stable fragment peak in the mass spectrum of 4-ethylheptane
Answers
The most stable fragment peak in the mass spectrum of 4-ethylheptane would have an m/z value of 57.
This is because the most stable fragment is the tertiary carbocation, which is formed by cleavage of the C-C bond between the 4th and 5th carbon atoms. The molecular formula of this fragment is C4H9, which has a molecular weight of 57.
Steps to determine m/x value1. Identify the most stable fragment in the molecule. In the case of 4-ethylheptane, this is the tertiary carbocation formed by cleavage of the C-C bond between the 4th and 5th carbon atoms.
2. Determine the molecular formula of the most stable fragment.
For 4-ethylheptane, this is C4H9. 3. Calculate the molecular weight of the most stable fragment using the molecular formula. For C4H9, this is 57. 4. The m/z value of the most stable fragment peak in the mass spectrum is equal to the molecular weight of the most stable fragment.
Therefore, the m/z value of the most stable fragment peak in the mass spectrum of 4-ethylheptane is 57.
Learn more about mass spectrum at
https://brainly.com/question/1698571
#SPJ11