In a 1.0×10^−6M solution of Ba(OH)2(aq) at 25 °C, arrange the species by their relative molar amounts in solution.
Greatest amount
least amount
Answer Bank: H2O, Ba(OH)2, OH^-, Ba^2+, H3O^+
Answers
Arranging the species by their relative molar amounts in a 1.0×10^−6M solution of Ba(OH)2(aq) at 25 °C:
Greatest amount: OH^-
Ba(OH)2
Ba^2+
H2O
H3O^-
In the given solution of Ba(OH)2(aq), the compound dissociates into its constituent ions, Ba^2+ and OH^-. The concentration of OH^- will be twice the concentration of Ba(OH)2 since each Ba(OH)2 molecule produces two OH^- ions. Therefore, OH^- will be present in the greatest amount.
Ba(OH)2 will be the next species in terms of molar amounts, followed by Ba^2+ since they are both present at half the concentration of OH^-. Water (H2O) does not participate in the chemical reaction and remains unchanged in terms of molar amounts. H3O^+ is not mentioned in the given compound Ba(OH)2 and is not present in this solution.
Therefore, based on the relative molar amounts, the arrangement of the species is as follows: OH^- (greatest amount), Ba(OH)2, Ba^2+, H2O, H3O^+ (least amount).
Learn more about molar: https://brainly.com/question/837939
#SPJ11
Related Questions
Titanium metal will react with liquid water to form solid titanium (IV) oxide and hydrogen gas. How many moles of titanium will be needed to fully react with 12.0 moles of water?
Answers
Answer:
Total we want 48 titanium to make 12.0moles
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
Assuming the pressure of a gas in a closed system is constant. If the temperature is increased, how can the system adjust to the change?
Answers
Answer:
Reducing the pressure in the system
what is the mass of a phosphorus pentachloride molecule ?
Answers
The mass of molecule will be 208.22 atomic mass units.
For determining the mass of a phosphorus pentachloride (PCl5) molecule, we need to consider the atomic masses of the individual elements and their respective quantities in the molecule.
The atomic masses of phosphorus (P) and chlorine (Cl) are approximately:
- Phosphorus (P): 30.97 atomic mass units (amu)
- Chlorine (Cl): 35.45 amu
Since there are five chlorine atoms in one molecule of phosphorus pentachloride, we multiply the atomic mass of chlorine by 5 to account for their total mass contribution.
Calculating the mass of a phosphorus pentachloride molecule:
Mass of phosphorus (P) = 30.97 amu
Mass of chlorine (Cl) = 35.45 amu
Total mass of phosphorus pentachloride (PCl5) molecule:
= Mass of phosphorus + Mass of chlorine (multiplied by 5)
= 30.97 amu + (35.45 amu x 5)
= 30.97 amu + 177.25 amu
= 208.22 amu
Therefore, the mass of a phosphorus pentachloride (PCl5) molecule is approximately 208.22 atomic mass units (amu).
To know more about mass refer here:
https://brainly.com/question/11954533?#
#SPJ11
in the distillation of a pure material, why does all of the pure material no vaportize once the boiling point is reched.
Answers
In the distillation of a pure material, all of the pure material not vaporize once the boiling point is reached because more heat would need to be added to the distillate in order to vaporize the liquid from its boiling point.
During distillation, the process of vaporizing a liquid and collecting the resulting vapor as a purified substance, it is important to consider the energy requirements involved.
When a liquid reaches its boiling point, it undergoes a phase change from the liquid phase to the gas phase. This phase change requires the input of energy in the form of heat. The heat breaks the intermolecular forces holding the liquid molecules together, allowing them to transition into the gas phase.
The heat required to vaporize a liquid is not solely determined by the boiling point. The heat required to convert a liquid into a gas is known as the heat of vaporization, and it varies depending on the substance.
When distilling a liquid, such as water, the heat of vaporization must be supplied to convert the liquid into vapor. This energy is absorbed by the liquid, and it is essential to provide continuous heating to maintain the distillation process.
As the liquid is heated and reaches its boiling point, vaporization begins. However, the rate at which the liquid vaporizes depends on the amount of heat being supplied. If the heat input is insufficient, the vaporization process will be slower, and not all of the liquid will vaporize at once.
To ensure the complete vaporization of a liquid during distillation, a sufficient amount of heat must be continuously applied to the system. This allows the heat of vaporization to be continually supplied to the liquid, facilitating the conversion of the entire liquid into vapor.
If the heat input is insufficient, the vaporization process will be slower, and the liquid may not vaporize all at once. Providing adequate and continuous heating is crucial to ensure the complete conversion of the liquid into vapor during distillation.
To know more about distillation here
https://brainly.com/question/31829945
#SPJ4
The following compounds will
decompose on heating except
A Ag2CO3.
B. CaCO3.
C. K2CO3
D. PbCO.
E. ZnCO3
Answers
AnsThe option C is correct
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If a 1.67 g sample of methane is burned at constant pressure, what will be the value of ∆H
Answers
Answer:
\(\Delta H=-92.7kJ\)
Explanation:
Hello there!
In this case, according to the given information, we can infer that 890 kJ of energy are released when 1 mole of methane is burned; however, to find the total heat when 1.67 grams are burned, we first need to calculate the moles in this mass of methane:
\(1.67gCH_4*\frac{1molCH_4}{16.04gCH_4}=0.104molCH_4\)
And thus, for calculating the resulting ∆H, we proceed as follows:
\(\Delta H=-890kJ/mol*0.104mol\\\\\Delta H=-92.7kJ\)
Regards!
Oxygen 16 is abundant and has 8 protons and 8 neutrons. oxygen-18 has two extra neutrons. these two forms are?
Answers
The two forms of oxygen 16 and oxygen 18 are isotopes.
An isotope is a type of atom that has the same number of protons as another atom of the same element but a different number of neutrons. An isotope is identified by the number of neutrons in the nucleus.Both oxygen-16 and oxygen-18 are isotopes of oxygen. Oxygen-16 has eight neutrons and eight protons in its nucleus. Oxygen-18 has ten neutrons and eight protons in its nucleus.
The difference in the number of neutrons between oxygen-16 and oxygen-18 gives them slightly different chemical and physical properties. For example, oxygen-18 is slightly heavier than oxygen-16, and it has a slightly lower boiling point. However, the two isotopes are chemically similar, and they can be used interchangeably in most chemical reactions.
To learn more about isotopes :
https://brainly.com/question/13604636
#SPJ11
How many moles of hydrogen do you need to react with 0.85 moles of nitrogen?
Answers
Answer:
6 moles
Explanation:
You have a 1:3 ratio between nitrogen gas and hydrogen gas
What do LEGOs and DNA have in common?
Answers
Answer:
They are both building blocks
Explanation:
DNA builds up life while legos build things
Learning Task No.5
identify the word or words being described by each statement.Chose your answer from box below.
1.It is the process of changing liquid to gas.
2.It is the process when water from plants evaporates.
3.It is the liquid part of the earth.
4.It is the cotinous movement of water on the earth's surface
5.The process of changing gas to liquid.
Please help ma to answer it
Thank you and goodbless
Answers
The appropriate term for each definition is given as follows:
It is the process of changing liquid to gas - evaporationIt is the process when water from plants evaporates - transpirationIt is the liquid part of the earth - hydrosphere It is the continous movement of water on the earth's surface - water cycleThe process of changing gas to liquid - condensationWhat is evaporation?Evaporation is the process of a liquid converting to the gaseous state while condensation is the conversion of a gas to a liquid.
Hydrosphere is all the liquid waters of the Earth, as distinguished from the land and the gases of the atmosphere.
Transpiration is the loss of water by evaporation in terrestrial plants, especially through the stomata of their leaves.
Water cycle is the continuous movement of water within the Earth and atmosphere.
Learn more about water cycle at: https://brainly.com/question/1151425
#SPJ1
Which structure represents nonane?
ОА.
Н
---
H-C-H
т
Н
ОВ.
H H
1 т
н-с-с-Н
1
Н Н
Ос.
Н
H
H H
т
H-C— С
1
H H
H
І
СЕН
1
Н
Н
Н
OD.
Н
1
Н
Н
H
T
Н
Н Н H
І т
H-C— С—
т
Н H Н
І
С
1
Н
1
H
І
Н
Н
С— г-н
1
Н H
Reset
Next
Answers
Explanation:
i don't understand what you wrote, but nonane is C9H20
A sample of helium gas is allowed to expand in a process that is adiabatic and quasistatic. As the gas cools from 105 degree C to 101 Degree C, it does 3.05 J of work on a piston. How many helium atoms are there in the sample?
Answers
According to the question, helium atoms are there in the sample is (3.05 J/P) / (R × (105 + 273.15 K)) × 6.02 x 10²³ atoms/mol.
What is helium atoms?Helium atoms are the second most abundant type of atom in the universe. They are the simplest of all atoms, consisting of only two protons and two neutrons. Helium atoms are extremely lightweight, with an atomic weight of only four, making them the second lightest element after hydrogen.
The number of helium atoms in the sample can be calculated using the ideal gas law: n = PV/RT
where n is the number of moles, P is the pressure, V is the volume, R is the ideal gas constant, and T is the temperature.
Since the process is adiabatic and quasistatic, the pressure and volume of the sample can be determined from the work done on the piston:
W = P(V2 - V1)
where W is the work done, V2 is the final volume, and V1 is the initial volume.
Since the work done is 3.05 J, the final volume is 3.05 J/P. The initial volume can be determined from the ideal gas law, using the initial temperature of 105°C and the number of moles (which is unknown).
n = PV1/RT1
where n is the number of moles, P is the pressure, V1 is the initial volume, R is the ideal gas constant, and T1 is the initial temperature.
Substituting the values into the ideal gas law, we can solve for the number of moles: n = (3.05 J/P) / (R × (105 + 273.15 K))
Once the number of moles is determined, the number of helium atoms can be calculated by multiplying by Avogadro's number.
N = n × 6.02 x 10²³ atoms/mol
Therefore, the number of helium atoms in the sample is:
N = (3.05 J/P) / (R × (105 + 273.15 K)) × 6.02 x 10²³ atoms/mol.
To learn more about helium atoms
https://brainly.com/question/2174015
#SPJ4
a scientist has an unknown substance and has identified that it is a carbohydrate. he is choosing another property to determine whether the substance is a monosaccharide or a polysaccharide. which of the following properties will be most useful?
Answers
To determine whether the unknown carbohydrate substance is a monosaccharide or a polysaccharide, the most useful property for the scientist to investigate would be the molecular size and structure of the substance.
Monosaccharides are simple sugars with smaller molecular structures, while polysaccharides are larger and consist of multiple monosaccharide units bonded together.
The combination of two monosaccharides results in a disaccharide, which can be classified as a disaccharide.
These are created through the blending of sugars. Since water is released after the reaction is finished, the process is called hydrolysis.
The glycosidic linkage joins two monosachrrides together. Maltose, sucrose, and lactose are a few prevalent examples.
A big molecule's chemical link is broken by the water molecule in a hydrolysis reaction, resulting in the formation of smaller molecules.
Comparatively speaking, a disaccharide is a larger molecule than a monosaccharide. Or disaccharide is twice as big as monosaccharide in another world. In order to create two smaller monosaccharides, water and disaccharide must react during the hydrolysis process.
Learn more about monosaccharide here
https://brainly.com/question/780562
#SPJ11
According to the VSEPR theory, which of the following factors determines the shape of a
molecule? Check all that apply.
Number of atoms attached to the central atom
Lone pair(s) on the central atom
Number of electrons in the first subshell
Number of neutrons in the nucleus
Answers
Answer:
B only
Explanation:
Using the VSEPR principle, the electron bond pairs and the lone pairs on the middle atom help us predict the structure of the molecule. The shape of a molecule is determined by the position of the nucleus and its electrons. The electrons and the nucleus settle in positions that minimize repulsion and maximize attraction.
The following factors determine the shape of a molecule Lone pair(s) on the central atom.
The correct answer is option B.
What is a lone pair on a central atom?In chemistry, a lone pair refers to a pair of valence electrons that are not shared with every other atom in a covalent bond and is once in a while known as an unshared pair or non-bonding pair. Lone pairs are located inside the outermost electron shell of atoms.
How do you find lone pairs on the central atom?A negatively charged carbon atom should right away let you know about a lone pair of electrons. In this situation, for the reason that carbon has the handiest 3 bonds and a poor fee, it should also have a lone pair. this can also be confirmed by means of the use of the method: FC= V – (N + B)
Learn more about negatively charged carbon atoms here: https://brainly.com/question/25633550
#SPJ2
There is always some between any surfaces that are in contact with each other
Answers
Answer:
Friction that is the answer pls brainlist me
During light-dependent reactions, carbon dioxide is converted to glucose.
Group of answer choices
True
False
Answers
Answer:The light-independent reactions use the ATP and NADPH from the light-dependent reactions to reduce carbon dioxide and convert the energy to the chemical bond energy in carbohydrates such as glucose.
Explanation:step-by-step
Answer:
FALSE
Explanation:
I just took the test and put true and it was wrong so it's false. Hope this helps and please mark brainliest!
Which of the following methods of preparation of amines results in an amine with one less carbon atom than the starting material?
a.reduction of a nitrile b.Gabriel synthesis from an alkyl halide
c.reductive amination of a ketone d.Hofman rearrangement of an amide
Answers
Hofmann rearrangement of an amide is the method of preparation of amines results in an amine with one less carbon atom than the starting material. The correct answer is d.
The Hofmann rearrangement is a reaction that converts a primary amide into a primary amine with one less carbon atom. The reaction is carried out by treating the amide with bromine and a base.
The bromine reacts with the amide to form an N-bromoamide. The base then removes a proton from the N-bromoamide to form an isocyanate. The isocyanate then rearranges to form a primary amine.
The Hofmann rearrangement is a useful method for preparing primary amines with one less carbon atom than the starting amide. The reaction is also used to prepare amines with specific functional groups.
For example, the Hofmann rearrangement can be used to prepare amines with hydroxyl groups or amino groups.
Therefore, the correct option is D, Hoffman rearrangement of an amide.
To know more about Hofmann rearrangement, refer here:
https://brainly.com/question/27749911#
#SPJ11
can i get help with this dont really get it

Answers
Answer:
Number 2 is Direction
Explanation:
The old answer for number four was incorrect
I apologize for the inconvenience but I do not have an answer that fits in the lines for number 4
Is the following equation balanced? Why or why not?
N, + 6H2 → 2NHO
Answers
Answer:
No
Explanation:
There is only one Nitrogen on the left side while there are 2 on the right. Also there is zero oxygen on the left side and two on the right. The amounts of hydrgen arent equal to each side either.
Someone please help with number 9.

Answers
Answer:
Its development and progression are usually linked to a series of changes in the activity of cell cycle regulators. For example, inhibitors of the cell cycle keep cells from dividing when conditions aren't right, so too little activity of these inhibitors can promote cancer.
Superficially, the connection between the cell cycle and cancer is obvious: cell cycle machinery controls cell proliferation, and cancer is a disease of inappropriate cell proliferation. Fundamentally, all cancers permit the existence of too many cells.
Explanation:
hope it helps
Los elementos que forman aniones con carga -1 y una capa externa estable de ocho electrones pertenecen al grupo de los:
Answers
Los elementos que forman aniones con carga -1 y una capa externa estable de ocho electrones pertenecen al grupo de los: halógenos.
Para alcanzar la estabilidad, los átomos siguen la regla del octeto: ganan, pierden o comparten electrones para tener 8 electrones en su capa externa (capa de valencia).
Los elementos del Grupo 17 de la Tabla Periódica, conocidos como halógenos, tienen 7 electrones en su capa de valencia. Así, tienden a ganar 1 electrón para completar su octeto. Como los electrones tienen carga negativa, al ganar 1 electrón los aniones quedan con una carga de -1.
Puedes aprender mas sobre la regla del octeto aquí: https://brainly.com/question/18892451
What is the molarity (mol/L) of a solution that is made from 0.25 mol of salt and 1.5 L of water?
Answers
The molarity of the solution is 0.1667 mol/L (or M).
To calculate the molarity of the solution, we need to know the number of moles of solute (the salt) and the volume of the solution. The number of moles is given as 0.25 mol, and the volume of the solution is 1.5 L. Therefore, we can use the following formula to calculate the molarity:
Molarity = moles of solute / volume of solution
Plugging in the values we have:
Molarity = 0.25 mol / 1.5 L
Molarity = 0.1667 mol/L
This means that for every liter of the solution, there are 0.1667 moles of salt dissolved in it. Molarity is a useful measure for describing the concentration of a solution because it takes into account both the amount of solute and the volume of the solution.
Knowing the molarity of a solution can help us to make accurate dilutions or to calculate the amount of solute needed to prepare a solution of a desired concentration.
For more question on molarity click on
https://brainly.com/question/30404105
#SPJ11
1-methylcyclohexanol reacts with hbr to form 1-bromo-1-methylcyclohexane. the mechanism for this reaction is likely to be an:
Answers
1-methylcyclohexanol reacts with hbr to form 1-bromo-1-methylcyclohexane. the mechanism for this reaction is likely to be an: S_N2 mechanism.
The reaction between 1-methylcyclohexanol and HBr likely proceeds via an S_N2 (nucleophilic substitution) mechanism. In this mechanism, the nucleophile (Br⁻) attacks the electrophilic carbon atom of the alkyl group (1-methylcyclohexyl carbon), resulting in the displacement of the leaving group (OH⁻).
The S_N2 mechanism is favored when the substrate is a primary or methyl alkyl halide, and in this case, 1-methylcyclohexanol is a primary alcohol. The S_N2 mechanism involves a concerted reaction in which the bond formation and bond breaking occur simultaneously.
It typically occurs with a strong nucleophile, in this case, the bromide ion (Br⁻), and in the presence of a polar solvent. The reaction proceeds in a one-step process, leading to the formation of 1-bromo-1-methylcyclohexane as the product.
learn more about Sn2 mechanism here:
https://brainly.com/question/30631335
#SPJ11
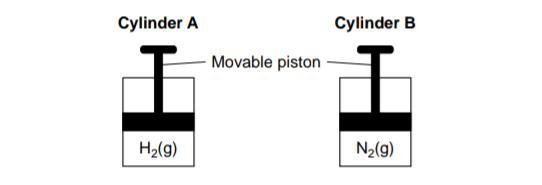
Cylinder A and Cylinder B are sealed, rigid cylinders with movable pistons. Each cylinder contains 500. milliliters of a gas sample at 101.3 kPa and 298 K. Cylinder A contains H2 and cylinder B contains N2. Explain, in terms of collisions between gas molecules and the walls of the container, why pushing the movable piston farther into cylinder B at constant temperature would increase the pressure of the N2 gas.
Answers
Answer:
The pressure of N₂ gas in cylinder B when compressed at constant temperature increases due to the increase in the frequency of collision between the gas molecules with themselves and with the wall of their container caused by a decrease in volume of the container.
Explanation:
Gas helps to explain the behavior of gases when one or more of either temperature, volume or pressure is varying while the other variables are kept constant.
In the gas cylinder B, the temperature of the given mass of gas is kept constant, however, the volume is decreased by pushing the movable piston farther into the cylinder. According to the gas law by Robert Boyle, the volume of a given mass of gas is inversely proportional to its pressure at constant temperature. This increase in pressure is due to the increase in the frequency of collision between the gas molecules with themselves and with the wall of their container caused by a decrease in volume of the container. As the cylinder becomes smaller, the gas molecules which were spread out further become more packed closely together, therefore, their frequency of collision increases building up pressure in the process.
In terms of collisions between gas molecules and the walls of the container, the reason why pushing the movable piston farther into cylinder B at constant temperature would increase the pressure of the N2 gas is due to the fact that;
The movement of the piston farther into the cylinder would lead to an increase in the number of collisions per unit area between the N2(nitrogen) molecules and the internal walls of the cylinder. The movement described above would lead to creation of greater pressure because the more the collisions, the higher the pressure.The image showing the two cylinders are missing and so i have attached it.
Read more at; https://brainly.com/question/24393357
a student is given an antacid tablet that weighs 5.6720 g. the tablet is crushed and 4.0460 g of the antacid is added to 200. ml of simulated stomach acid. it is allowed to react and then filtered. it is found that 25.00 ml of this partially neutralized stomach acid required 11.0 ml of a naoh solution to titrate it to a methyl red end point. it takes 27.4 ml of this naoh solution to neutralize 25.00 ml of the original stomach acid. how much of the stomach acid (in ml) would be neutralized by the full 5.6720 g tablet?
Answers
The full antacid tablet would neutralize 1.41 L of stomach acid.
let's calculate the concentration of the simulated stomach acid. We added 4.0460 g of antacid to 200 ml of stomach acid, so the concentration is:
C1 = (4.0460 g / 200 ml) * (1 mol / 102.09 g) = 0.0197 M
1 mol of HCl reacts with 1 mol of NaOH
So the number of moles of HCl neutralized by the NaOH solution is:
n = C2 * V2 = (0.1000 mol/L) * (27.4 mL / 1000 mL) = 0.00274 mol
n_antacid = 5.6720 g / 102.09 g/mol = 0.0555 mol
so the number of moles of HCl neutralized by the antacid tablet is:
n_HCl = 0.5 * n_antacid = 0.0278 mol
V_HCl = n_HCl / C1 = 0.0278 mol / 0.0197 mol/L = 1.41 L
Concentration refers to the amount of a solute dissolved in a given amount of solvent or solution. It is usually expressed in units of molarity (moles per liter) or weight per volume (grams per liter). There are various methods for measuring concentration, including titration, spectrophotometry, and gravimetry. These methods allow chemists to accurately determine the amount of solute in a solution and adjust the concentration as needed.
Concentration plays a crucial role in many chemical reactions and processes. It can affect the rate of a reaction, the yield of a product, and the properties of a solution. For example, a higher concentration of reactants can increase the rate of a reaction, while a higher concentration of a solute can increase the boiling or melting point of a solution.
To learn more about Concentration visit here:
brainly.com/question/10725862
#SPJ4
Which statement describes a chemical property of
aluminum?
(1) Aluminum has a density of 2.698 g/em at STP.
(2) Aluminum reacts with sulfuric acid.
(3) Aluminum conducts an electric current.
(4) Aluminum is malleable.
Answers
Answer:
2 aluminum reacts with sulfuric acid
What is the atomic number of Gold
Answers
Match the name of each gas law to the properties it compares. Boyle's law Charles's lawGay-Lussac's lawA) Pressure and volume B) Temperature and volume C) Pressure and temperature
Answers
Answer:
Boyle's law: A) Pressure and volume.
Charles's law: B) Temperature and volume.
Gay-Lussac's law: C) Pressure and temperature.
Explanation:
Hello,
In this case, since Boyle's law study the relationship between pressure and volume at constant temperature as an inversely proportional one, we have:
Boyle's law: A) Pressure and volume.
Next, since the Charles' law study the relationship between the volume and the temperature at constant pressure as a directly proportional one, we have:
Charles's law: B) Temperature and volume.
Then, since the Gay-Lussac's law study the relationship between the pressure and the temperature at constant volume as a directly proportional one as well, we have:
Gay-Lussac's law: C) Pressure and temperature.
Best regards.
Answer:
D, B, E
Explanation:
Determine the [h3o ] of a 0.180 m solution of benzoic acid.
Answers
Using benzoic acid's acidity constant we can calculate that [H₃O⁺] = 3.34 x 10⁻³ M.
To answer this, we must write the expression for benzoic acid (BzOH) acidity constant (Ka):
\(Ka = \frac{[H_{3}O^{+} ][BzO^{-} ]}{[BzOH]}\)
[H₃O⁺] - molarity of H₃O⁺ ions
[BzO⁻] - molarity of benzoate ions
[BzOH] - molarity of undissociated benzoic acid
The acidity constant for benzoic acid is 6.3 * 10⁻⁵.
If the initial concentration of benzoic acid is 0.180 M, then the molarity of undissociated BzOH will be 0.180 - X, and [H₃O⁺] = [BzO⁻] = X.
We can plug all of this into the expression to get:
\(6.3 *10^{-5} = \frac{X^{2} }{0.180 - X}\)
We can transform this expression:
X² = 1.134 * 10⁻⁵ - 6.3 * 10⁻⁵ * X
X² + 6.3 * 10⁻⁵ * X - 1.134 * 10⁻⁵ = 0
When we solve for X, we get
X = 3.34 x 10⁻³ M
So [H₃O⁺] = 3.34 x 10⁻³ M
You can learn more about acidity constants here:
brainly.com/question/4363472
#SPJ4