In order for rocks to be classified as igneous, the rocks must
Answers
In order for rocks to be classified as igneous, the rocks must be developed from molten lava.
What is rock?Rock is a naturally occurring and cohesive conglomerate of one or more minerals in geology. These aggregates are the fundamental unit of the solid Earth and often form identifiable and mappable volumes. Rocks are typically classified into three broad groups based on the processes which resulted in their creation.
Igneous rocks are those that developed from molten lava. The heat within the earth's mantle turns this substance liquid. When magma rises to the earth's surface, it is known as lava. When lava cools, it forms rocks like tuff and basalt.
Therefore, in order for rocks to be classified as igneous, the rocks must be developed from molten lava.
To learn more about rock, here:
https://brainly.com/question/29767269
#SPJ1
Related Questions
a supramolecular sorting hat: stereocontrol in metal–ligand self assembly by complementary hydrogen bonding
Answers
The term "supramolecular sorting hat" refers to a concept in chemistry where metal-ligand self-assembly is controlled by complementary hydrogen bonding.
In this context, "supramolecular" means the assembly of molecules or ions into larger, more complex structures, and "sorting hat" is an analogy to the magical hat from Harry Potter that sorts students into different houses based on their characteristics. The "stereocontrol" in this process refers to the ability to control the spatial arrangement of the resulting supramolecular structures. This can be achieved by using ligands that have specific geometries and hydrogen bonding capabilities, which can selectively bind to metal ions and guide the assembly process.
The "complementary hydrogen bonding" refers to the formation of hydrogen bonds between the ligands and the metal ions. Hydrogen bonding is a type of intermolecular interaction where a hydrogen atom bonded to an electronegative atom (such as oxygen or nitrogen) is attracted to another electronegative atom. In summary, the term "supramolecular sorting hat: stereocontrol in metal-ligand self assembly by complementary hydrogen bonding" describes a process in chemistry where the self-assembly of metal-ligand complexes is controlled by complementary hydrogen bonding interactions, leading to the formation of specific supramolecular structures. This concept allows for the selective arrangement of molecules based on their geometries and hydrogen bonding capabilities.
To know more about supramolecular visit:
https://brainly.com/question/14495313
#SPJ11
Which of the elements listed below appear as polyatomic molecules in their standard states? Select all that apply.OxygenFluorineHydrogenNeonBeryllium
Answers
Oxygen Hydrofluoric Acid Nitric Oxide All of these compounds are polyatomic.
Why are molecules polyatomic in nature?Three or more atoms form stable structures to form polyatomic molecules (bound state). The molecular formula, which reveals the precise number of constituent atoms, serves as a means of distinguishing different compounds.
How is polyatomic distinguished?Only a few of the periodic table's elements have a second letter that is lowercase after their capital initial. As a result, you will recognize a polyatomic ion if it contains two capital letters together. Two diatomic gases, nitrogen and oxygen, make up its major constituents.
To know more about polyatomic visit:-
https://brainly.com/question/25055220
#SPJ4
when a scientist uses many specific observations to construct a general scientific principle, the scientist is using reasoning.true or false
Answers
The statement is true, because when a scientist uses many specific observations to construct a general scientific principle, the scientist is using reasoning.
This is an example of inductive reasoning, which is a form of logical thinking that uses specific observations to create general principles.
The importance of scientists lies in their ability to develop testable explanations and predictions about the universe based on scientific evidence.
Learn more about scientist:
https://brainly.com/question/5019543
#SPJ4
Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
+ 3 02
2 Fe2O3
How many moles of Iron II Oxide can be produced from 347.7 L of Oxygen gas at a temperature
of 74.3 °C and a pressure of 294.5 kPa?
Round Answers to 0.01 decimals

Answers
Answer:
2.0
Explanation:
How do you find the frequency of a recorder?
Answers
Answer:
Modern recorders are most commonly pitched at A=440 Hz, but among serious amateurs and professionals, other pitch standards are often found. For the performance of baroque music, A=415 Hz is the de facto standard, while pre-Baroque music is often performed at A=440 Hz or A=466 Hz.
Explanation:
which of the following factors affect the magnitude of the lattice energy for an ionic compound? select all that apply.
Answers
The factors that affect the magnitude of the lattice energy for an ionic compound include the charge of the ions, the size of the ions, and the distance between the ions. Therefore, all of the following factors affect the magnitude of the lattice energy:
1. Charge of the ions
2. Size of the ions
3. Distance between the ions
The factors that affect the magnitude of the lattice energy for an ionic compound include:
1. Ionic charge: Higher charges on the ions lead to a greater electrostatic attraction between them, resulting in a larger lattice energy.
2. Ionic size: Smaller ions have stronger interactions due to their closer proximity, leading to a higher lattice energy.
These two factors are the primary determinants of lattice energy for ionic compounds.
Lattice energy is a measure of the strength of the electrostatic forces between ions in an ionic compound. It is defined as the amount of energy required to completely separate one mole of an ionic solid into its constituent ions in the gas phase, with the ions at an infinite distance from each other.
Lattice energy depends on several factors, including the charges of the ions, the distance between them, and the arrangement of the ions in the crystal lattice. The greater the charges of the ions and the closer they are to each other, the higher the lattice energy. Additionally, lattice energy is inversely proportional to the distance between the ions, so as the distance between the ions decreases, the lattice energy increases.
The lattice energy can be calculated using the Born-Haber cycle, which is a series of steps that describes the formation of an ionic compound from its constituent elements. The steps involve the formation of gaseous atoms or ions, the transfer of electrons to form ions, and the formation of the solid ionic compound.
Lattice energy is an important property of ionic compounds because it affects their physical and chemical properties. Compounds with higher lattice energies tend to have higher melting and boiling points, be more soluble in polar solvents, and have greater stability in solution. Understanding the lattice energy of an ionic compound can provide insight into its reactivity and behavior in different environments.
To know more about lattice energy visit:
https://brainly.com/question/31730061
#SPJ11
Which of the following next-generation batteries would be good choices based on the future availability of its elements?
Al-ion
Mg-ion
Li-ion
Sn-ion
Answers
Based on the future availability of its elements, the best choice for a next-generation battery would be Mg-ion.
Magnesium is the eighth most abundant element in the Earth's crust, and it is relatively inexpensive to extract and process.
Additionally, Mg-ion batteries have the potential to be more energy-dense than Li-ion batteries, which could make them a more attractive option for electric vehicles and other applications where weight and space are important considerations.
Al-ion and Sn-ion batteries are also promising technologies, but they are still in the early stages of development. Li-ion batteries are the most widely used type of rechargeable battery today, but they have some drawbacks, such as the fact that lithium is a relatively rare element.
As a result, Mg-ion batteries could be a good alternative to Li-ion batteries in the future.
Magnesium is the most abundant element used in any of these types of batteries. This makes it a good choice for a next-generation battery, as it is likely to be more available and less expensive than other elements.
To know more about batteries, refer here:
https://brainly.com/question/31604572#
#SPJ11
Can you show me the answer and explain?

Answers
Answer:
C
Explanation:
looking at a periodic table X is fluorine and Y is potassium
Fluorine is in group 7 and forms a 1- charge (which gains electrons) and potassium is in group 1 and forms a 1+ charge (which loses electrons)
Fluorine (X) has an electronic structure of 2,7 and needs to gain an electron from Potassium (Y) to have a full outer shell and potassium has an electronic structure of 2,8,8,1 so needs to lose an electron to have a full outer shell as well. This means that the electron that potassium (Y) has lost is given away to fluorine (X), so both elements become stable.
This is known as ionic bonding where metals (like potassium) lose electrons and non-metals (like fluorine) gain electrons to become more stable, forming ions
Any further clarification let me know
Can someone help me Please?
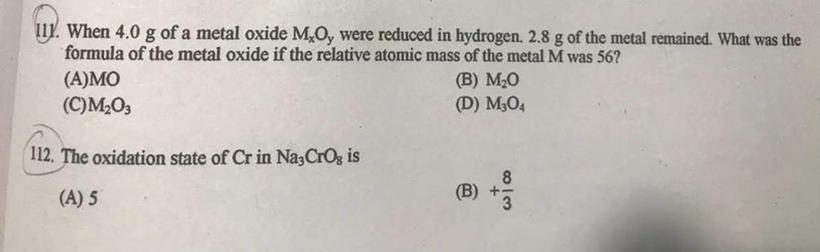
Answers
Answer:
111. C . M2O3
112. A. 5
Please help :/ || 10 pts || will mark brainlest ||
In which equation is carbon dioxide a product?
Group of answer choices
CH4 + 2O2 --> CO2 + 2H2O
CO2 --> C + O2
2H2O --> 2H2 + O2
6CO2 + 6H2O --> C6H12O6 + 6O2
Answers
Answer:
CH4 + 2O2 --> CO2 + 2H2O
Explanation:
"CO2" can be written as carbon dioxide
450 J of heat are added to a 50 g block of lead. What is the resulting
temperature change? c for lead is 0.13 J/g(degC)
Answers
Answer:
69.2°C
Explanation:
Recall the heat energy formula
q = cmΔT
where q = heat energy ( measured in joules ) , c = specific heat , m = mass and ΔT = change in temperature.
We are given that:
The heat energy is 450J The mass is 50g The specific heat is 0.13 J/g(degC)Given this we want to find the temperature change.
First we want to define our variables.
Again recall that heat energy (q) = 450J , mass(m) = 50g and specific heat (c) = 0.13 J/g(degC)
Now that we have defined our variables we plug in the values of the variables into q = cmΔT and solve for the undefined variable
q = cmΔT
q = 450 , m = 50, c = 0.13
450 = (50) x (0.13) x (ΔT)
multiply 50 and .13
450 = 6.5 x (ΔT)
divide both sides by 6.5
69.2 = ΔT
The change in temperature was 69.2°C
14. A force acts for 0.2 second on a body of mass 80 kg at rest and produces a velocity of 10 ms¹.Find the magnitude of the force.
Answers
The magnitude of the force acting on the body is 4000 Newtons.
To find the magnitude of the force, we can use Newton's second law of motion, which states that the force applied to an object is equal to the product of its mass and acceleration.
The given information includes the mass of the body (80 kg) and the resulting velocity (10 m/s). However, since the time duration (0.2 seconds) is also provided, we can use it to calculate the acceleration of the body.
The formula to calculate acceleration is:
Acceleration = Change in Velocity / Time
The change in velocity can be calculated by subtracting the initial velocity (which is 0 m/s as the body is at rest) from the final velocity:
Change in Velocity = Final Velocity - Initial Velocity
Change in Velocity = 10 m/s - 0 m/s
Change in Velocity = 10 m/s
Now, we can calculate the acceleration:
Acceleration = Change in Velocity / Time
Acceleration = 10 m/s / 0.2 s
Acceleration = 50 m/s²
Finally, we can calculate the magnitude of the force using Newton's second law:
Force = Mass x Acceleration
Force = 80 kg x 50 m/s²
Force = 4000 N
Therefore, the magnitude of the force acting on the body is 4000 Newtons.
for more questions on force
https://brainly.com/question/8106035
#SPJ11
i like HOW NO ONE IS HELPING ME LIKE WHAT OMG IM LOSING POINTS FOR NOTHING

Answers
Answer:
Combustion reaction
Explanation:
Combustion reactions always form water and CO2.
g sio2 is a(n) covalent network solid. ki is a(n) -- solid. ti is a(n) -- solid. c6h12o6 is a(n) -- solid.
Answers
The kinds of the solids are;
SiO2 - Covalent network solid
C6H12O6 - Covalent solid
KI - Ionic solid
What is a covalent network solid?
A covalent network solid, often referred to as a network covalent solid or just a network solid, is a category of solid material in which the atoms that make up the material are strongly covalently linked to one another, forming an extended three-dimensional network structure.
Covalent network solids are kept together by a dense network of covalent bonds, as opposed to molecular or ionic solids, which are held together by weaker intermolecular forces or ionic interactions, respectively.
Learn more about covalent network solid:https://brainly.com/question/30458552
#SPJ4
What is the volume (in liters at STP) of 70.0 g of carbon monoxide, CO?
Answers
The volume that is occupied by the gas is obtained as 56 L.
What is the volume of the CO?We know that from the Avogadro's law, the volume that can be occupied by one mole of a gas is obtained as 22.4 L. This implies that we have to find the number of moles in the 70 g of the CO and then obtain the corresponding volume by simple proportion.
Number of moles of CO = 70.0 g/28 g/mol
= 2.5 moles
If 1 mole of the gas occupies 22.4 L
2.5 moles of the gas occupies 2.5 * 22.4/1 mole
= 56 L
Learn more about volume of a gas:https://brainly.com/question/12357202
#SPJ1
A teacher divides her class into groups and assigns each
group the task of measuring the mass of the same object
three times. The teacher already knows that the mass of
the object is 25 g.
Based on the results each group records, which group makes the most
precise measurements of the object?
O
A. Group C: 32.1 g, 35.0 g, 25.0 g
O
B. Group B: 25.5 g, 25.0 g, 24.8 g
O
C. Group A: 28.5g, 28.4 g, 28.5 g
O D. Group D: 20.0 g, 25.0 g, 30.09
Answers
Answer:
Group C
Explanation:
Precision is how close the values are to each other
a student determined the empirical formula of magnesium oxide by heating a known mass of magnesium in air and weighing the product after the magnesium had burned. which factors would result in her calculated formula having a higher ratio of magnesium to oxygen than 1:1? i. not all the magnesium reacted ii. some of the product escaped before it was weighed iii. some of the product was magnesium nitride, mg3n2
Answers
All the magnesium do not reacted and some of the product was magnesium nitrite, Mg₃N₂ are the factors . Option B is correct.
The empirical formula, which is defined as the ratio of subscripts of the smallest possible whole number of the elements in the formula, is the simplest formula for a compound.
Mg + O₂( air) ⇒ MgO.
assume mass of magnesium [Mg]= w₁ g
mass of the product [MgO] = w₂ g
Thus, mass of oxygen in MgO = w₂-w₁
= x g
Now, to explain the experimental result, which indicates a higher Mg to oxygen ratio rather than a 1:1 ratio, this x g is less than w₁ g.
i) x< w₁ If all of the w₁ is not reacted, less w₂ is formed, and the ratio will be greater than 1:1.
ii) If a side effect occurs and some magnesium nitride is heated in the air, [Mg₃N₂] also formed.
Mg + N₂( air) ⇒ Mg₃N₂ ,
Because it contains both the product MgO and Mg₃N₂, x g will yield a higher Mg ratio.
iii) Option II is incorrect because both MgO and Mg₃N₂ are white amorphous solids that are stale and inert. So it can't be evaded during assortment.
Learn more about Empirical formula:
brainly.com/question/1603500
#SPJ1
Complete question as follows:
A student determined the empirical formula of magnesium oxide by heating a known mass of magnesium in air and weighing the product after the magnesium had burned. Which factors would result in her calculated formula having a higher ratio of magnesium to oxygen than 1:1? I 1. Not all the magnesium reacted II. Some of the product escaped before it was weighed III. Some of the product was magnesium nitrite, Mg, N,
A. I and II only
B. I and Ill only
C. Il and Ill only
D. I, II and III
Identify the product from the reaction of nitrobenzene with iron and hydrochloric acid, followed with base. Chlorobenzene aniline benzene 1,3-dinitrobenzene 1,4-dinitrobenzene
Answers
The product from the reaction of nitrobenzene with iron and hydrochloric acid, followed with base is Aniline.
The product from the reaction of nitrobenzene with iron and hydrochloric acid, followed with base is Aniline.What is nitrobenzene?Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a yellow, oily liquid that is structurally similar to benzene, with one nitro group replacing one hydrogen atom. The nitro group is responsible for the compound's characteristic aroma and toxic properties.What is the reaction of nitrobenzene with iron and hydrochloric acid, followed with base?Nitrobenzene is reduced to aniline with iron and hydrochloric acid. The reaction is exothermic and occurs at room temperature. In the presence of hydrochloric acid, iron is oxidized to ferrous chloride (FeCl2) as nitrobenzene is reduced to aniline, according to the following reaction:Nitrobenzene + 6Fe + 12HCl → Aniline + 6FeCl2 + 4H2OThe aniline is subsequently separated from the reaction mixture by washing with a base solution such as sodium hydroxide, which removes any ferrous chloride present in the solution, leaving pure aniline.
Learn more about nitrobenzene here:
https://brainly.com/question/28182533
#SPJ11
Determine the mass (in g) of each NaCl solution that contains 1.7 g of NaCl.
0.068% NaCl by mass
Answers
By mass, the mass of the NaCl solution containing 1.7 g of NaCl at 0.068% concentration is approximately 2500 g.
To determine the mass of the NaCl solution containing 1.7 g of NaCl with a concentration of 0.068% NaCl by mass, you can use the following formula:
mass of solution = (mass of solute) / (percentage concentration / 100)
Here, the mass of solute (NaCl) is 1.7 g, and the percentage concentration is 0.068%.
mass of solution = (1.7 g) / (0.068 / 100)
mass of solution = 1.7 g / 0.00068
mass of solution ≈ 2500 g
So, the mass of the NaCl solution containing 1.7 g of NaCl at 0.068% concentration by mass is approximately 2500 g.
More on mass: https://brainly.com/question/30692810
#SPJ11
Step 1: treatment with NaBH_4 Add curved arrows for the first step, Treat Na^+ as a spectator ion. Step 2: treatment with D_2O Add curved arrows for the second step. Do not show Na^+ counter ion.
Answers
The reaction occurs via a nucleophilic substitution mechanism. The D_2O molecule acts as the nucleophile, attacking the carbon atom of the CH_3CH_2OBH_3 molecule.
Step 1:
NaBH_4 + CH_3CH_2OH → CH_3CH_2OBH_3 + NaOH
Step 2:
CH_3CH_2OBH_3 + D_2O → CH_3CH_2OD + BH_3OH
Step by explanation in detail
Step 1:
NaBH_4 + CH_3CH_2OH → CH_3CH_2OBH_3 + NaOH
The reaction occurs via a nucleophilic substitution mechanism. The Na^+ cation acts as a spectator ion and does not take part in the reaction. The BH_4- anion acts as the nucleophile, attacking the carbon atom of the CH_3CH_2OH molecule. This causes the C-O bond to break and the BH_3 group to form a bond with the carbon atom. This results in the formation of the CH_3CH_2OBH_3 molecule and the NaOH molecule.
Step 2:
CH_3CH_2OBH_3 + D_2O → CH_3CH_2OD + BH_3OH
The reaction occurs via a nucleophilic substitution mechanism. The D_2O molecule acts as the nucleophile, attacking the carbon atom of the CH_3CH_2OBH_3 molecule. This causes the C-B bond to break and the D atom to form a bond with the carbon atom. This results in the formation of the CH_3CH_2OD molecule and the BH_3OH molecule.
learn more about nucleophilic substitution here
https://brainly.com/question/16172504
#SPJ4
Describe the position of each part of an atom in the bohe model
Answers
Explanation:
Hey, there!!
The parts are an atom are:
Electrons Protons NeutronsGenerally, Protons and Neutrons lies in the centre of an atom. After that the shells made outer are covered by electrons. We keep 2 electrons in 1st shell, 8 electrons in 2nd, 18 electrons in 3rd shell and so on. The positions of neutron and protons are always in the nucleus and electrons are in the orbits or shell.
Hope it helps....
I NEED HELP ASAP IM ON AN EXAM
ONLY CORRECT ANSWERS WILL MARK BRAINLIST

Answers
ANSWER:
Applied research, because it is solving a problem.
EXPLANATION:
Pure research refers to the systematic study of life aimed at fuller knowledge or a deeper understanding of the theoretical aspects of a phenomenon. On the other hand, applied research is a systematic, empirical study undertaken to generate knowledge for problem-solving in a very broad sense.
a scientist is testing an unknown metal sample. he burns the sample and measures the spectrum produced. he concludes that the metal is sodium because of the characteristic bright yellow lines he observes. what is the general term for the type of technique he is performing?
Answers
The answer to the question indicates that the scientist is engaged in spectroscopy.
What is sodium's simple definition?(SOH-dee-um) A mineral required by the body to maintain the proper balance of body fluids. Table salt and many processed meals also contain sodium. The body may retain water if it is oversalted.
What functions does salt serve?In some nuclear plants, sodium is employed as a heat exchanger.It is also utilized in the chemical industry as a reagent. Instead, sodium salts are so much more advantageous than sodium alone. Sodium chloride is the most prevalent sodium compound (common salt). It is used to flavor meals and de-ice roadways in the winter.
To know more about Sodium visit :
https://brainly.com/question/29327783
#SPJ4
Which type of weathering has the greatest impact in areas with warm climates and lots of rainfall?
Answers
Answer:
In general, hot wet climates accelerate chemical weathering while cold dry climates accelerate physical weathering. Although the rate of weathering depends on the type of rock, rocks in tropical climates experience the highest rates of weathering because of the combination of high heat and heavy rainfall.
zeolites theoretically can be made magnetic by adding sodium ion to them. T/F ?
Answers
The statement "zeolites theoretically can be made magnetic by adding sodium ion to them" is true.
Zeolites are a group of minerals that are highly porous and capable of exchanging ions. This property enables zeolites to absorb and store sodium ions, and when exposed to an external magnetic field, the stored sodium ions create their own magnetism, causing the zeolite to become magnetic.
The process of creating a magnetic zeolite begins with a zeolite powder, which is typically heated to very high temperatures to create a larger surface area for the sodium ions to bind to. Then, a solution of sodium ions is introduced, which is then absorbed into the zeolite.
The zeolite is then placed in a magnetic field, which causes the absorbed sodium ions to align in the direction of the field, creating a magnetic property in the zeolite. To summarize, it is true that zeolites can be made magnetic by adding sodium ions to them.
This is accomplished by exposing the zeolite powder to high temperatures and introducing a solution of sodium ions, which are then absorbed and exposed to a magnetic field that causes the ions to align and create magnetism in the zeolite.
To know more about zeolites refer here:
https://brainly.com/question/28588461#
#SPJ11
Examine Table 10.3 and list the compounds you think have hydrogen bonds. Explain why.


Answers
Answer
Ethanol and Ethylene Glycol
Procedure
Hydrogen bonding occurs when hydrogen forms a molecular bond with a highly electronegative element such as Oxygen, Nitrogen, and Fluorine. Based on the structure of the molecules we can see that ethane does not contain the previously mentioned elements, therefore it will not form hydrogen bonds. Dimethyl ether has an oxygen atom located in the middle of the molecule, making it difficult to form a bond with other dimethyl ether molecules.
Lastly, Ethanol and Ethylene Glycol possess OH groups which are free to interact with similar groups via hydrogen bonding. Additionally, these last compounds exhibit higher boiling points, which can indicate a stronger intermolecular bonding, which is a characteristic of hydrogen bonding.
what are plasmas properties?
Answers
Answer:Plasma is highest energy state of matter.It consists of electrons,protons and neutral particles.
Explanation:(1) Plasma has a very high electrical conductivity .
(2) The motion of electrons and ions in plasma produces it's own electric and magnetic field
(3)It is readily influenced by electric and magnetic fields .
(4)It produces it's on electromagnetic radiations.
Humid air contains a large amount of water vapor this makes it more difficult for water particles to evaporate from a dish of water will humid conditions speed up or slow down the rate of transpiration?
Answers
Answer:
Slow down
Explanation:
In humid conditions plant transpiration rates decrease.
what is the formula for radiant to chemical energy to make glucose
Answers
The radiant energy that is absorbed by the plant in the process of photosynthesis is stored in the plant in the form of chemical energy contained in sugars.
What is photosynthesis?The term photosynthesis has to do with the process by which there is a combination of water and carbon dioxide in the plant such that the products that are formed are sugar and oxygen. The oxygen is released back into the environment.
We know that radiant energy from the sun is what acts as the catalyst that makes the reaction possible. The combination occurs in the chloroplasts of the plant cells .
Learn more about photosynthesis:https://brainly.com/question/1388366
#SPJ1
Which indicator would show a pH change from 6 to 7?
A. Red litmus indicator
B. Methyl red indicator
C. Phenol red indicator
D. Blue litmus indicator
Answers
Answer:
c
Explanation:
1. litmus paper is used when showing a change between a greater range in ph levels - so A and D are automatically a no.
2. methyl red is used to show a range in ph levels between 4.8-6
3. Option C is the only one left so im going to assume its C because its definitely not A, B, or D