in which orbital does an electron add to in chlorine to form an octet?
Answers
In chlorine, the electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁵. To form an octet, chlorine needs to gain one additional electron. The electron will add to the 3p orbital, specifically occupying the 3p⁶ orbital.
By adding an electron to the 3p orbital, chlorine achieves a stable electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶, which corresponds to a complete octet with eight valence electrons.
This completes the filling of the 3p orbital with a total of six electrons. The addition of this electron allows chlorine to fulfill the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons.
To know more about the electron configuration refer here :
https://brainly.com/question/14283892#
#SPJ11
Related Questions
PLEASE HELPPPP I WILL GIVE BRAINLIST

Answers
The possible values of the quantum numbers are:
Orbital shape quantum number l: 0, 1, 2, 3, 4, or 5.
Magnetic quantum number m: -l to +l.
Spin quantum number, s: +1/2 or -1/2.
What are the azimuthal, magnetic, and spin quantum numbers of the valence electrons in an atom with principal quantum number, n = 6?For the principal quantum number n = 6, the possible values of the azimuthal (orbital shape) quantum number l are 0, 1, 2, 3, 4, and 5.
The magnetic quantum number m can have values ranging from -l to +l, inclusive.
The spin quantum number, s can be either +1/2 or -1/2.
So, the valence electrons in an atom with principal quantum number n = 6 can have the following quantum numbers:
Azimuthal quantum number, l can be 0, 1, 2, 3, 4, or 5.
Magnetic quantum number, m can range from -l to +l, inclusive.
Spin quantum number, s can be either +1/2 or -1/2.
Learn more about quantum numbers at: https://brainly.com/question/2292596
#SPJ1
in order for a thermonuclear fusion reaction of two deuterons (21h ) to take place, the deuterons must collide and each must have a velocity of about 1×106m/s.
Answers
True. In order for a thermonuclear fusion reaction of two deuterons (2H or D) to occur, the deuterons must indeed collide, and each deuteron must have a velocity of approximately 1x\(10^6\) m/s.
Thermonuclear fusion reactionFor a thermonuclear fusion reaction of two deuterons (2H) to occur, each deuteron must collide with a velocity of approximately 1x10^6 m/s.
This high velocity is needed to overcome the electrostatic repulsion between the positively charged deuterons and allow the strong nuclear force to bring them close enough for fusion to happen.
The collision between the deuterons can then result in the formation of a helium-3 nucleus and a high-energy neutron. Achieving such velocities is a challenge in controlled fusion due to the need for high temperatures and confinement techniques.
More on fusion reactions can be found here: https://brainly.com/question/28020465
#SPJ4
Does the proton affect the mass of an atom ?
Answers
Answer:
Yes a proton affects the mass of an atom.
7) Methyl alcohol (methanol) liquid is stored in a vessel. Its vapor is inerted with nitrogen to a total pressure of 2-inch of water gauge. (Assume a temperature of 25°C; 1 atm = 406.8 inches of water = 760 mmHg; LOLMeOH = 7.3% and UOLMeOH = 81%) a. Determine the saturated vapor pressure of methanol in the vessel in mmHg.(3 Marks ans: 125.9 mmHg) b. Determine the volume percent concentration of methanol in the vapor. (3 Marks ans: 16.5% MetOH) Hint: use Dalton law: Meto psat/Pabs =
Answers
The saturated vapor pressure of methanol in the vessel is 125.9 mmHg (rounded off to 3 significant figures). The volume percent concentration of methanol in the vapor is 73.2% (rounded off to 3 significant figures).
a. Saturated vapor pressure of methanol in the vessel in mmHg: The vapor pressure of methanol (MeOH) in the vessel can be determined using Dalton's law of partial pressures. MetOH psat = MetOH Pabs
Here, MetOH Pabs is the pressure exerted by the vapor of methanol in the vessel. It is equal to the total pressure in the vessel minus the partial pressure of the inert gas (nitrogen). Pabs = 2 inch of water gauge = 2 x 25.4 mm of water gauge / 1 inch of water gauge = 50.8 mm of water gauge
Total pressure in mmHg = Pabs x 760 mmHg / 406.8 inch of water gauge = 94.8 mmHg
Partial pressure of nitrogen = 2 inch of water gauge = 50.8 mm of water gauge
Partial pressure of methanol = MetOH Pabs MetOH psat = 126 mmHg (at 25°C)
Therefore, the saturated vapor pressure of methanol in the vessel is 125.9 mmHg (rounded off to 3 significant figures).
b. Volume percent concentration of methanol in the vapor: The volume percent concentration of methanol in the vapor can be determined using the ideal gas law and the Dalton's law of partial pressures. V = nRT / PV = volume of the vapor (L)n = number of moles of methanol gas R = gas constant T = temperature (K)P = pressure (atm)
Let's assume that the total volume of the vapor in the vessel is 1 L and the temperature is 25°C (298 K).
The number of moles of nitrogen gas (N2) in the vapor can be determined using the Dalton's law of partial pressures. N2 Pabs = Pabs - MetOH PabsN2 Pabs = 50.8 mm of water gaugeN2 Pabs = 50.8 mm of water gauge x 1 atm / 760 mmHg = 0.067 atmN2 V = nRT / PN2N2 V = (0.067 atm x 1 L) / (0.082 L atm/K mol x 298 K)N2 V = 0.0022 mol
The number of moles of methanol gas (MeOH) in the vapor can be determined using the ideal gas law. MetOH Pabs = MetOH psatMetOH Pabs = 125.9 mm Hg MetOH Pabs = 125.9 mmHg x 1 atm / 760 mmHg = 0.165 atmMetOH V = nRT / PMetOH V = (0.165 atm x 1 L) / (0.082 L atm/K mol x 298 K)MetOH V = 0.006 mol
The volume percent concentration of methanol in the vapor can be determined using the following equation.
Volume percent concentration of MeOH = MetOH V / (N2 V + MetOH V) x 100%
Volume percent concentration of MeOH = 0.006 mol / (0.0022 mol + 0.006 mol) x 100%
Therefore, the volume percent concentration of methanol in the vapor is 73.2% (rounded off to 3 significant figures).
To know more about saturated vapor pressure refer to:
https://brainly.com/question/30447018
#SPJ11
A student was asked to determine the activity of four unknown metals W,X,Y and, Z.

Answers
Answer:
BBBBBBBBBBBBBBBBBBBBBBBBBBBBBBBB
The activity of four unknown metals based on their chemical changes is option B.
What is a chemical change?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical change,here:
https://brainly.com/question/23693316
#SPJ3
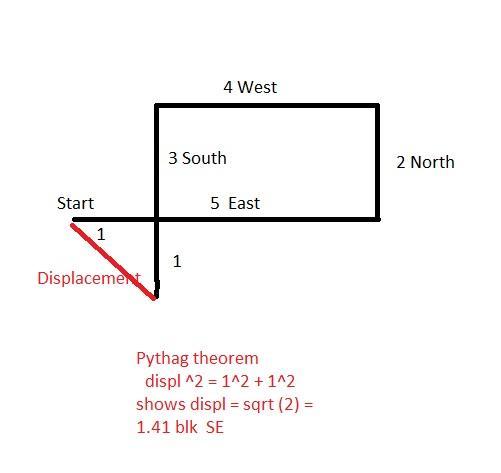
What is the total displacement of a student who walks 5 blocks East,2 blocks north,4 blocks west, and then 3 blocks south?
Answers
Explanation:
See image
Which element in Group 18 of the Periodic Table has the highest lonization energy?
Answers
all the elements in group 18 are Nobel gases or inert gases . all the elements such as neon , helium, argon etc. ,their outermost shell is completely filled . The noble gases have the largest ionization energies, reflecting their chemical inertness
why does fluorine have the highest electronegativity
Answers
Answer:
Because it only needs one more electron to get to a full valence shell (8), so it really wants it and is pulling other electrons in. It also has to do with needing one more electron to fill the 2p shell. It is a small element which means its electrons are pulled tightly to the nucleus.
Hope this helps!
Explanation:
30. Which two notations represent atoms that are isotopes of the same
element?
A) 31 Sn and 30°Sn
B) 581 Sn and 13 Sn
C) 3°O and LOF
D) 19 Cl and 18K
Answers
Answer: 581 Sn and 13 Sn
Explanation:
581 Sn and 13 Sn are both Sn which are the same element with different masses. Isotopes are same element with different masses and the elements can have different masses because of different number of neurons
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
REALLY NEED HELP
Aqueous Precipitation Reactions:
1. Complete and balance the following chemical equations for double replacement reactions. Make sure you indicate if a solid precipitate is formed.
2. Write the lonic Equation and the net ionic equation for each
Answers
Answer:You're dealing with a double replacement reaction in which two soluble ionic compounds react in aqueous solution to form an Insoluble solid.
As you know, soluble ionic compounds exist as ions in solution. In your case, a solution of sodium carbonate,
Na
2
CO
3
, will contain
Na
2
CO
3(aq]
→
2
Na
+
(aq]
+
CO
2
−
3(aq]
Likewise, a solution of calcium chloride,
CaCl
2
, will contain
CaCl
2(aq]
→
Ca
2
+
(aq]
+
2
Cl
−
(aq]
When these two solutions are mixed, the calcium cations,
Ca
2
+
, will apir up with the carbonate anions,
CO
2
−
3
, and form the Insoluble calcium carbonate,
CaCO
3
, which precipitates out of solution.
The complete ionic equation, which features all the ions that are present in solution, will look like this
2
Na
+
(aq]
+
CO
2
−
3(aq]
+
Ca
2
+
(aq]
+
2
Cl
−
(aq]
→
CaCO
3(s]
⏐
⏐
↓
+
2
Na
+
(aq]
+
2
Cl
−
(aq]
The net ionic equation, for which spectator ions are omitted - remember that spectator ions are those ions located on both sides of the equation - will look like this
CO
2
−
3(aq]
+
Ca
2
+
(aq]
→
CaCO
3(s]
⏐
⏐
↓
To get the overall balanced chemical equation, simply look at he complete ionic equation and group the ions back to an ionic compound
Na
2
CO
3
2
Na
+
(aq]
+
CO
2
−
3(aq]
+
CaCl
2
Ca
2
+
(aq]
+
2
Cl
−
(aq]
→
CaCO
3(s]
+
2
NaCl
2
Na
+
(aq]
+
2
Cl
−
(aq]
Notice that you have two sodium cations and two chloride anions on the products' side, which is why you have
2
NaCl
.
Na
2
CO
3(aq]
+
CaCl
2(aq]
→
CaCO
3(s]
⏐
↓
+
2
NaCl Hope it helps :)
The word "precipitation reaction" refers to a chemical reaction that takes place within an aqueous solution as two ionic bonds join, resulting in the production of an insoluble salt.
What do aqueous precipitation reactions entail?When dissolved substances react, one (or even more) solid products are produced, which is known as a precipitation reaction. These kinds of reactions, which are also occasionally known as double displacement, twofold replacement, or metathesis reactions, frequently involve the exchange or ions between ionic substances in water-based solutions.
Which two precipitate reactions are examples of them?Examples include the interaction of calcium chloride (CaCl2) with potassium hydroxide (KOH), which produces the insoluble salt calcium hydroxide. Silver https://brainly.com/question/11527546(AgCl), an insoluble salt, is produced by the interaction of silver nitrate (AgNO3) with sodium chloride (NaCl).
To know more about ionic bonds visit:
https://brainly.com/question/11527546
#SPJ1
What percent of the energy that comes from the Sun to the Earth is...reflected______warm land and water_______photosynthesis_________
Answers
Approximately 30% of the energy that comes from the Sun to the Earth is reflected back into space. This phenomenon is known as albedo, and it is primarily influenced by the reflective properties of clouds, ice, and the Earth's surface. These surfaces reflect sunlight rather than absorbing it, resulting in a cooling effect on the planet.
A significant portion of the Sun's energy, around 47%, warms the land and water on Earth. When sunlight reaches the Earth's surface, it is absorbed by the land, water bodies, and vegetation, causing an increase in temperature. This absorbed energy plays a crucial role in driving various atmospheric and oceanic processes, including weather patterns, ocean currents, and the water cycle.
The remaining 23% of the Sun's energy is utilized by photosynthesis, the process through which plants and some microorganisms convert sunlight into chemical energy. This energy powers the growth and development of plants, allowing them to synthesize sugars and other organic compounds necessary for their survival. Photosynthesis also plays a vital role in maintaining the balance of atmospheric gases, as plants absorb carbon dioxide and release oxygen, contributing to the regulation of the Earth's climate.
In summary, while 30% of the Sun's energy is reflected, approximately 47% warms the land and water, and the remaining 23% is used for photosynthesis, sustaining life on Earth and shaping our planet's climate systems.
To know more about Sun's energy click this link-
https://brainly.com/question/13922537
#SPJ11
Mol to Mol Stoichiometry
Potassium reacts with cobalt III iodide to produce potassium iodide and cobalt. How many moles of potassium iodide are produced when 0.55 moles of cobalt III iodide reacts?
A.0.89 moles
B.3.5 moles
C.1.7 moles
Answers
Answer:
c
Explanation:
I really don't know and i just guessed
molecules move in random directions when heated in a heat engine, and because of the lack of uniformity in direction of molecular movement, true or false
Answers
The statement "molecules move in random directions when heated in a heat engine, and because of the lack of uniformity in the direction of molecular movement" is true.
When a heat engine is heated, molecules absorb heat energy and their kinetic energy increases. The kinetic energy of molecules causes them to move around. However, this movement is not uniform, and the molecules move in random directions.
A heat engine is a device that converts thermal energy into mechanical energy. Heat engines operate on the principle of thermodynamics.
They work by taking in thermal energy from a high-temperature reservoir, converting some of it into mechanical energy, and then releasing the remaining thermal energy to a low-temperature reservoir.The internal combustion engine in a car, the steam engine in an old locomotive, and the turbine in a power plant are all examples of heat engines. They all convert heat energy into mechanical energy to perform work.
To learn more about engine visit;
https://brainly.com/question/31140236
#SPJ11
How are S waves and surface waves similar?
Both arrive after P waves.
Both compress the ground.
Both travel through liquids.
Both produce minimal ground motion.
I will give out brainliest
Answers
Answer:
The answer to this question is A. Both arrive after P waves
Explanation:
Because since P waves always come first surface and S waves come after.
PLEASE
Why don't the particles in a solid move past one another?
A. They have no motion and cannot even vibrate in place.
B. They don't have enough energy to overcome the attractions
between them.
C. They bounce off the walls of their container and back into place.
D. They move independently of one another.
Answers
Answer:
B. They don't have enough energy to overcome the attractions between them.
Answer:They don't have enough energy to overcome the attractions
between them.
Explanation:
A. They have no motion and cannot even vibrate in place.
B. They don't have enough energy to overcome the attractions
between them.
C. They bounce off the walls of their container and back into place.
D. They move independently of one another.

Gravity is a (n) _____________ between objects and depends on an object's size and their distance apart.
Answers
Gravity is a (n) force between objects and depends on an object's size and their distance apart.
Gravitational force is the attractive that exist between all object with mass an object with mass attracts another object with mass the magnitude of the force is directly proportional to the masses of the two objects and inversely proportional to the square of the distance between the two objects
Know more about objects
https://brainly.com/question/27786016
#SPJ1
At 20°C a gas has a volume of 16.00 L. What will the volume be at 175.0 °C?
Answers
The volume of the gas at 175.0 °C will be 24.50 Litres
What will the volume of the gas be at 175.0 °C?Charles's law states that "the volume occupied by a definite quantity of gas is directly proportional to its absolute temperature.
It is expressed as;
V₁/T₁ = V₂/T₂
Where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
To use this formula, we need to convert the temperatures to Kelvin by adding 273.15 to them:
T1 = 20°C + 273.15 = 293.15 K
T2 = 175.0°C + 273.15 = 448.15 K
Substituting the values into the formula, we get:
16.00 L / 293.15 K = V2 / 448.15 K
Solving for V2, we get:
V2 = 24.50 L
Therefore, the final volume is 24.50 L.
Learn more about Charles's law here: https://brainly.com/question/23122443
#SPJ1
Alkanes contain the maximum possible amount of hydrogen, they are known as being __________. What one word completes the sentence?
Answers
Alkanes contain the maximum possible amount of hydrogen, they are known as being saturated hydrocarbon.
What is alkanes?The easiest category of hydrocarbons would be the alkanes. They exclusively possess hydrogen as well as carbon. So every hydrogen atom produces one link, while each carbon atom generates four.
What is hydrocarbon?When all of a hydrocarbon's carbon-carbon bonds possess single bonds, the hydrocarbon was said to have been saturated. A hydrocarbon would be an organic substance with only carbon as well as hydrogen as its components.
Therefore, alkanes contain the maximum possible amount of hydrogen, they are known as being saturated hydrocarbon.
To know more about alkanes and hydrocarbons.
https://brainly.com/question/16995585
#SPJ3
5
Use the equation Moles = Mass/Mołar Mass (Mr) to calculate the following: To make 3 moles of
Sodium (Na), how much mass do I need to weigh out?
A: 699g
B: 6.9g
C: 69g
D: 96g
Answers
Answer:
c
Explanation:
Molar mass of Na=23g/mol
amount of mole(n)=3
mass=?
therefore,n=m/M
3mol=m/23g/mol
m=23g/mol ×3mol
m=69g.
1) What is the ground state electron configuration for Oxygen (O)?
2) What is the excited state electron configuration for Oxygen (O)
Help please
Answers
Answer:
1)Oxygen atoms have 8 electrons and the shell structure is 2.6. The ground state electron configuration of ground state gaseous neutral oxygen is [He]. 2s2. 2p4 and the term symbol is 3P2.
Explanation:
2)If the element were to become excited, the electron could occupy an infinite number of orbitals. However, in most texts the example will be the next available one. So for oxygen, it might look like this: 1s22s22p33s1 - where the valence electron now occupies the 3s orbital in an excited (i.e. not ground) state
What is the percent composition of Cesium and Fluorine in CsF?a.50% Cesium, 50% Fluorineb.73% Cesium, 27% Fluorinec.87% Cesium, 13% Fluorined.23% Cesium, 77% Fluorine
Answers
Answer
c. 87% Cesium, 13% Fluorine
Explanation
From the periodic table, the atomic masses of F = 18.998403 g/mol and Cs = 132.90545 g/mol.
So the molar mass of CsF = 132.90545 + 18.998403 = 151.903853 g/mol
\(\begin{gathered} Percent\text{ }composition\text{ }of\text{ }Cesium=\frac{Atomic\text{ }mass\text{ }of\text{ }Cs}{Molar\text{ }mass\text{ }of\text{ }CsF}\times100\% \\ \\ Percent\text{ }composition\text{ }of\text{ }Cesium=\frac{132.90545}{151.903853}\times100\%=87\% \end{gathered}\)\(\begin{gathered} Percent\text{ }composition\text{ }of\text{ }Fluorine=\frac{Atomic\text{ }mass\text{ }of\text{ }F}{Molar\text{ }mass\text{ }of\text{ }CsF}\times100\% \\ \\ Percent\text{ }composition\text{ }of\text{ }Cesium=\frac{18.998403}{151.903853}\times100\%=13\% \end{gathered}\)Therefore, the percent composition of Cesium and Fluorine in CsF is 87% Cesium, 13% Fluorine
What is the symbol for temperature?
m
q
T
J
Answers
Answer:
T
Explanation:
m is mass
q is heat
J is energy
is the colour chrome green produced by the same type of electronic transition that causes the colour of chrome yellow?
Answers
No, the color chrome green is not produced by the same type of electronic transition that causes the color of chrome yellow.
The color chrome green is produced by the presence of chromium(III) ions in a complex, such as chromium(III) oxide hydroxide. The green color arises from the absorption of specific wavelengths of light by the chromium(III) ions, which are in a particular electronic configuration. The absorption of light in this case is due to the d-d transition, which involves the excitation of an electron from one d orbital to another within the chromium(III) ion.
On the other hand, the color of chrome yellow, also known as lead(II) chromate, is a result of a different type of electronic transition. Chrome yellow exhibits a yellow color due to the presence of lead(II) chromate ions, which absorb specific wavelengths of light. In this case, the absorption of light is attributed to the charge transfer transition between the lead(II) and chromate ions.
The colors chrome green and chrome yellow are produced by different types of electronic transitions. Chrome green involves d-d transitions within chromium(III) ions, while chrome yellow involves charge transfer transitions between lead(II) and chromate ions.
Learn more about electronic transition here: brainly.com/question/29221248
#SPJ11
which of the following statements about liquids is true? the boiling point of a solution is dependent solely on the atmospheric pressure over the solution. droplet formation occurs because of the higher stability associated with increased surface area. liquid rise within a capillary tube because of the small size lowers the effective atmospheric pressure over the surface of the liquid. substances that can form hydrogen bonds will display lower melting points than predicted from periodic trends. london dispersion forces arise from a distortion of the electron clouds within a molecule or atom.
Answers
The true statement about liquids among the options given is that substances that can form hydrogen bonds will display lower melting points than predicted from periodic trends. Option D is correct.
This is because the presence of hydrogen bonds allows for stronger intermolecular forces between molecules, which makes it easier for them to break apart and enter a liquid state at lower temperatures than would otherwise be expected based on their molecular weight and other properties. The other statements are not true: the boiling point of a solution is affected by factors other than atmospheric pressure, droplet formation is caused by the lower stability associated with increased surface area, liquid rise in a capillary tube due to surface tension rather than lowered atmospheric pressure, and London dispersion forces arise from temporary fluctuations in electron density rather than a permanent distortion.
When a metal and a melt are brought into contact with the tube and an accessory after heating, capillary molten solder occurs. Because there is a little distance between the wall of the tube and that of the fitting, capillary action causes the molten metal to rise and extend in any direction. When the metal cools, this results in a totally hermetic union.
Learn more about capillary molten here
https://brainly.com/question/15177419
#SPJ11
The Complete question is
Which of the following statements about liquids is true?
A. The boiling point of a solution is dependent solely on the atmospheric pressure over the solution.
B. droplet formation occurs because of the higher stability associated with increased surface area.
C. liquid rise within a capillary tube because of the small size lowers the effective atmospheric pressure over the surface of the liquid.
D. substances that can form hydrogen bonds will display lower melting points than predicted from periodic trends.
E. london dispersion forces arise from a distortion of the electron clouds within a molecule or atom.
a. Match the following terms to their definitions. (2 points)
A. Mole ___ The number of items in a mole
B. Molarity ___ Mass of 1 mole of something
C. Molar mass ___ 6.02 × 1023 items
D. Avogadro's number ___ Concentration of a dissolved substance
Answers
A. Mole - The number of items in a mole
B. Molarity - Concentration of a dissolved substance
C. Molar mass - Mass of 1 mole of something
D. Avogadro's number - 6.02 × 10^23 items
A mole is a unit used in chemistry to represent a specific number of items, which is approximately 6.02 × 10^23. It is often used to count atoms, molecules, ions, or other particles.
Molarity, on the other hand, is a measure of the concentration of a dissolved substance in a solution. It is expressed as the number of moles of the solute divided by the volume of the solution in liters.
Molar mass refers to the mass of one mole of a substance. It is calculated by summing up the atomic masses of all the atoms in a molecule or formula unit. Molar mass is expressed in grams per mole.
Avogadro's number is a fundamental constant in chemistry and physics. It represents the number of items (atoms, molecules, ions, etc.) in one mole of a substance. Avogadro's number is approximately 6.02 × 10^23 items per mole.
In summary, the terms can be matched as follows:
A. Mole - The number of items in a mole,
B. Molarity - Concentration of a dissolved substance,
C. Molar mass - Mass of 1 mole of something,
D. Avogadro's number - 6.02 × 10^23 items.
To know more about mole visit:
https://brainly.com/question/29367909
#SPJ11
4. Solid lead has a density of 11.34 g/cm³. Molten (liquid) lead has a density of 10.66 g/cm³. If you
melted a 510 g piece of lead, how much more volume will it take up?
Answers
The amount of volume that the piece of lead will take up would be 2.87 cm³
How to find the volume ?The difference in these volumes will give us the additional volume taken up by the melted lead.
Density (ρ) is defined as mass (m) divided by volume (V), or ρ = m/V. Rearranging the formula to find the volume, we get V = m/ρ.
First, let's find the volume of solid lead (V solid):
V solid = m solid / ρ solid
V solid = 510 g / 11.34 g/cm³
V solid ≈ 44.98 cm³
Now, let's find the volume of liquid lead (V_liquid):
V liquid = m liquid / ρ liquid
V liquid = 510 g / 10.66 g/cm³
V liquid ≈ 47.85 cm³
Finally, let's find the difference in volume:
ΔV = V liquid - V solid
ΔV ≈ 47.85 cm³ - 44.98 cm³
ΔV ≈ 2.87 cm³
Find out more on volume at https://brainly.com/question/20410293
#SPJ1
which metal is the most easily oxidized?
A. highly active metal
B. moderately active metal
C.slightly active metal
D.an inactive metal
Answers
Answer:
B. Moderately active metal
Explanation:
Given the balanced equation representing a reaction:
Ni(s) + 2HCl(aq) - NiCl(aq) + H2(9)
In this reaction, each Ni atom
1.
loses 1 electron
2.
loses 2 electrons
لما
gains 1 electron
4
gains 2 electrons
Submit
Answers
Answer: loses 2 electrons
Explanation:
Ni Atom loses 2 electrons , Option 2 is the correct answer.
What is an Electron ?Electron is a subatomic particle (denoted by the symbol e⁻)
or whose electric charge is negative one elementary charge.
Electrons belong to the first generation of the lepton particle family and are generally thought to be elementary particles because they have no known components or substructure.
The electron has a mass that is approximately 1/1836 that of the proton.
Ni(s) + 2HCl(aq) - NiCl(aq) + H₂(g)
This is an oxidation-reduction (redox) reaction:
2 H⁺ + 2 e⁻ → 2 H (reduction)
Ni - 2 e⁻ → Ni ²⁺ (oxidation)
HCl is an oxidizing agent, Ni is a reducing agent.
Therefore Ni Atom loses 2 electrons , Option 2 is the correct answer.
To know more about Electron
https://brainly.com/question/12001116
#SPJ2
Describe the benefits of ultrasound to a Grignard reaction
Answers
Ultrasound can be used as a tool to enhance the reaction rate and yield in a Grignard reaction. Some of the benefits of ultrasound to a Grignard reaction are:
Accelerated reaction rate: Ultrasonic waves generate acoustic cavitation bubbles that collapse and create high energy hotspots, resulting in localized heating and pressure waves. These cavitation bubbles can lead to the formation of free radicals or other reactive species, which can accelerate the Grignard reaction rate. This can result in faster reaction times and higher yields.
Improved mixing: Ultrasonic waves also create microstreaming and turbulence within the reaction mixture, which can enhance the mixing of reactants and improve the homogeneity of the reaction. Improved mixing can lead to better mass transfer and more efficient collisions between reactant molecules, which can further enhance the reaction rate and yield.
Reduced reaction time: The use of ultrasound in a Grignard reaction can reduce the reaction time, as the high-energy cavitation bubbles can accelerate the reaction. This can result in faster reaction times, which can be particularly advantageous for large-scale reactions.
Improved selectivity: Ultrasound can also improve the selectivity of the Grignard reaction by promoting the formation of the desired product and suppressing the formation of unwanted byproducts. This is likely due to the enhanced mixing and localized heating that occurs during ultrasonic irradiation.
Learn more about Ultrasound
https://brainly.com/question/30363405
#SPJ4