ir contains 360 ppmv of carbon dioxide. your class room air ducts supplies air such that the concentration of co2 never exceeded 600 ppm, when you may feel tired or sleepy. your room is (600 cubic meter) designed for 40 student capacity and consider room temperature 22 oc and 0.976 atm. on average human produces 900 grams of co2 per day. answer the following (a) what will be the required flow rate to achieve this design at steady state. (b) consider the room was fiiled with ambient air before the start of class, when the room carbon dioxide concentration reaches 400 ppm with the flow rate obtained in part a? (c) when steady state will be achieved?
Answers
(a) Required flow rate to achieve this design at steady state:
The required flow rate is calculated using the following formula:
Flow rate (mass/time) =
Concentration difference x Volume of air x Density x Cp Concentration difference
= 600 - 360 = 240 ppmv
Volume of air = 600 m3Density of air
= 1.2 kg/m3Cp = 1 kJ/kg-K Flow rate (mass/time)
= 240 x 600 x 1.2 x 1 / 3600 Flow rate (mass/time) = 4.8 kg/h
Therefore, the required flow rate to achieve this design at steady state is 4.8 kg/h. (b) When the room carbon dioxide concentration reaches 400 ppm with the flow rate obtained in part a:The initial carbon dioxide concentration was 360 ppmv,
which means the initial mass of carbon dioxide in the room was:
Mass = Concentration x Volume x Density Mass
= 360/1,000,000 x 600 x 1.2Mass = 0.2592 kg
When the room's carbon dioxide concentration reaches 400 pp mv, the mass of carbon dioxide in the room would be: Mass = Concentration x Volume x Density Mass
= 400/1,000,000 x 600 x 1.2Mass = 0.288 kg
Therefore, the mass of carbon dioxide that has been added to the room is: Mass added
= 0.288 - 0.2592Mass added
= 0.0288 kg
The time taken to reach this concentration can be calculated as follows:
Mass flow rate = Flow rate x Density Mass flow rate
= 4.8 x 1.2Mass flow rate
= 5.76 kg/h Time
= Mass added / Mass flow rate Time
= 0.0288 / 5.76Time = 0.005 hours
Therefore, it will take 0.005 hours or 18 seconds for the carbon dioxide concentration to reach 400 ppmv.
(c) When steady state will be achieved? Steady state is achieved when the amount of carbon dioxide added to the room is equal to the amount of carbon dioxide removed from the room. The amount of carbon dioxide added to the room was calculated in part (b) to be 0.0288 kg.
The amount of carbon dioxide produced by humans in the room can be calculated as follows: Number of students = 40Mass of carbon dioxide produced per day = 900 g Mass of carbon dioxide produced per hour = 900 / 24Mass of carbon dioxide produced per hour = 37.5 g/h Total mass of carbon dioxide produced = 40 x 37.5
To know more about carbon dioxide concentration refer to:
https://brainly.com/question/29461791
#SPJ11
Related Questions
If the pressure of a gas sample is quadrupled and the absolute temperature is doubled, by what factor does the volume of the sample change
Answers
Answer:
The new volume of the sample is halved.
Explanation:
Data obtained from the question include the following:
Initial volume (V1) = V
Initial temperature (T1) = T
Initial pressure (P1) = P
Final pressure (P2) = quadrupled = 4P
Final temperature (T2) = doubled = 2T
Final volume (V2) =?
Thus, we can obtain the new volume of the same by using the combined gas equation as shown below:
P1V1 /T1 = P2V2 /T2
P × V/T = 4P × V2/2T
Cross multiply
T × 4P × V2 = P × V × 2T
Divide both side by T × 4P
V2 = (P × V × 2T) / (T × 4P)
V2 = V/2
V2 = ½V
Therefore, the new volume of the sample is halved .
food choices in all societies are driven by an inborn preference for what food flavors?
Answers
There may be some universal preferences for basic taste sensations, food choices in societies are a complex blend of biological, cultural, and individual factors.
Food choices in all societies are not solely driven by an inborn preference for specific food flavors. While taste preferences do have a biological component, food choices are influenced by a complex interplay of various factors, including cultural, environmental, social, and individual factors.
That being said, there are certain taste preferences that are commonly observed across many societies. These are often referred to as the basic taste sensations, which include sweet, sour, salty, bitter, and umami (savory). These basic tastes are thought to be universally recognized and experienced by humans due to their biological significance.
The preference for sweet flavors is believed to be innate and is associated with the perception of energy-rich foods. This preference may have evolutionary roots as sweet tastes are typically found in ripe fruits, which are a good source of energy. Similarly, the aversion to bitter tastes is thought to be an innate response to potentially toxic substances that often have a bitter taste.
Click the below link, to learn more about Food Choices:
https://brainly.com/question/13456232
#SPJ11
Wxy =EFG write another valid congruency statement
Answers
EFG = Wxy is one possible congruence statement that is equivalence to and valid for Wxy = EFG.
What congruency statements are legitimate?If one triangle can be positioned on top of the other so that they coincide, two triangles are said to be congruent (fit together). As a result, congruent triangles are precise replicas of one another and, when assembled, have sides and angles that match.
What constitutes a triangular congruency statement that is true?The triangles are congruent if two sides of one bmatch two sides of another triangle, and if the included angles are also congruent. Using labels: Triangle ABC is congruent to triangle DEF if in triangles ABC and DEF, AB = DE, AC = DF, and angle A = angle D.
To know more about equivalence visit:-
https://brainly.com/question/30464075
#SPJ1
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
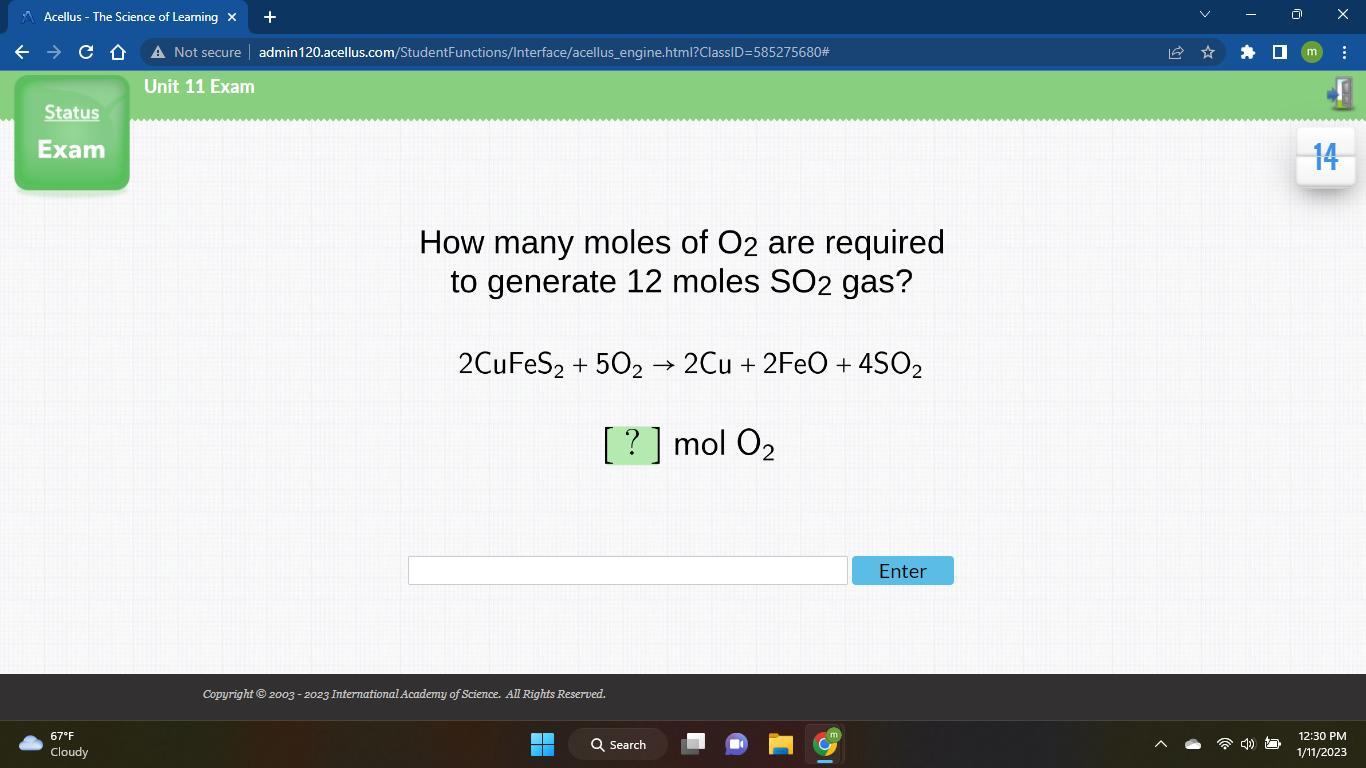
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
Explain:
(a) Ethanol should be rectified after distillation.
Answers
Answer:
Ethanol (boiling point 78°C) and methanol (boiling point 65°C) are two miscible liquids. There is only a small difference in their boiling points. These two liquids are separated from their mixture by fractional ths distillation.
When vapours of the mixture pass through the fractionating column, repeated liquefaction and vapourisation take place. Subsequently, the vapours of low boiling methanol enter the condenser from the fractionating column, condense to liquid and get collected in the round bottomed flask first. Similarly, ethanol with higher boiling point can be collected later in another round bottomed flask.
The remnants of an ancient fire in a cave in Peru showed a decay rate of 8.0 counts per minute per gram of carbon. Assuming that the decay rate in freshly cut wood is 12.7 counts per minute per gram of carbon, calculate the age of the remnants. The half life of 614C is 5730 years.
Answers
The age of the remnants is calculated as 3807 years.
What is the half life?The half life is the time taken for only half of the number of the radioactive atoms to remain.
Half life of carbon - 14 = 5730 years.
Initial count rate = 8.0 counts per minute per gram
Count rate at time t = 12.7 counts per minute per gram of carbon
Since;
0.693/t1/2 =2.303/t log (N/No)
0.693/5730 = 2.303/t log (12.7/8)
1.21 * 10^-4 = 2.303/t * 0.2
t = 2.303 * 0.2/1.21 * 10^-4
t = 0.4606/1.21 * 10^-4
t = 3807 years
Learn more about carbon-14:https://brainly.com/question/4206267
#SPJ1
If the mixture in question 1 is in a 3.0 Liter container at 34 °C, what mass (in grams)of oxygen is present?If oxygen was 0.472 atm
Answers
Answer
The mass (in grams) of oxygen present = 1.79 grams.
Explanation
Given
Volume, V = 3.0 L
Temperature, T = 34 °C = (34 + 273.15 K) = 307.15 K
Pressure, P = 0.472 atm
What to find:
The mass (in grams) of oxygen present.
Step-by-step solution:
Step 1: Calculate the moles of oxygen present.
Using the ideal gas law:
\(PV=nRT\)R is the molar gas constant = 0.0820574 L•atm/mol•K.
\(\begin{gathered} 0.472\text{ }atm\times3.0\text{ }L=n(0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K) \\ \\ n=\frac{0.472\text{ }atm\times3.0\text{ }L}{0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K} \\ \\ n=0.056\text{ }mol \end{gathered}\)The moles of oxygen present is 0.056 mol.
Step 2: Convert 0.056 mol oxygen to mass in grams.
The molar mass of oxygen gas = 31.998 g/mol
Using the mole formula below, the mass of oxygen can be calculated as follows:
\(\begin{gathered} Moles=\frac{Mass}{Molar\text{ }mass} \\ \\ \Rightarrow Mass=Moles\times Molar\text{ }mass \\ \\ Mass=0.056\text{ }mol\times31.998\text{ }g\text{/}mol \\ \\ Mass=1.791888\text{ }g\approx1.79\text{ }grams \end{gathered}\)The mass (in grams) of oxygen present = 1.79 grams.
Explain why an organism dies if the respiratory and circulatory system 'paused' for a while.
Answers
Answer:
Without the respiratory system your blood would be useless. The circulatory and respiratory systems work together to circulate blood and oxygen throughout the body
Explanation:
calculate the relative atomic mass of a metal M given that 0.65g of metal forms 1.61g of a sulfate of formula M
Answers
The relative atomic mass of the metal, M is 65 g/mol.
What are the mole ratio of the metal and the sulfate?The mole ratio of the metal and the sulfate is calculated from the mass of the metal and the sulfate present in the compound.
Mass of the compound = 1.61 g
Mass of the metal, M, in the compound = 0.65 g
Mass of sulfate = mass of compound - the mass of metal
Mass of sulfate = 1.61 - 0.65
Mass of sulfate = 0.96 g
The formula of the compound is MSO₄
1 mole of the metal combines with 1 mole of sulfate
Moles of sulfate in the compound = mass / molar mass
Molar mass of sulfate = 96 g/mol
Moles of sulfate = 0.96 / 96
Moles of sulfate = 0.01 moles
Hence, the moles of metal in the compound is 0.01 moles
The relative atomic mass of the metal = 0.65 / 0.01
The relative atomic mass of the metal = 65 g/mol
Learn more about relative atomic mass at: https://brainly.com/question/23093395
#SPJ1
Complete question:
Calculate the relative atomic mass of a metal M given that 0.65g of the metal forms 1.61g of sulfate of formula MSO₄. (S = 32, O = 16).
rank the species (carbonate chloride iodate and sulfate) from most to least soluble
Answers
The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride. Carbonates are the most soluble.
What is solubility?The maximum amount of a material that may dissolve in another is known as its solubility. A saturated solution is created when a solvent can dissolve its maximum quantity of solute before reaching equilibrium. A supersaturated solution results when extra solutes are dissolved past its equilibrium solubility point under specific circumstances.
Dissolution is the action of disintegrating. In contrast to the speed of solution, which specifies how rapidly a molecule dissolves in a solvent, solubility is not a feature of matter. The order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
Therefore, the order of solubility from most to least can be given as carbonate>sulfate>iodate>chloride.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ1
A solution contains 90 mL of methanol, 18 mL of propanol, and 2 mL of diethyl ether. Which is the solvent in this solution
solution?
O methanol
O propanol
O diethyl ether
O water
Answers
Answer:
Methanol
Explanation:
The solvent in this solution is methanol
What is Solvent?Solvent is a substance in which the solute is dissolved.polar and non polar are two types of solvent.
What are Polar solvents and Non Polar solvents?A solvent which has dipole moments are known as polar solvents.A polar solvent can dissolve ions and other polar compounds.A non polar solvent is a solvent which does not have dipole moments.
What is solute?A substance that dissolves in solution is called solute.When two liquids are mixed to form a solution, the solute is the species that is present in the smaller ratio.
Why Methanol is the solvent in this solution?Methanol is the component that has the greatest volume compared to other solvents. It means that it is found in a greater proportion. So methanol is the solvent in this solution.
Hence the other options are incorrect.
Learn more about Solvents below
https://brainly.com/question/25326161
#SPJ2
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Hydrogen peroxide decomposes to form water and oxygen. The uncatalysed reaction has activation energy of 86 kJ/mol. The activation energy value in the presence of acetanilide is 112 kJ/mol and in the presence of MnO2 it is 49 kJ/mol. What conclusion can you draw from the above observation ?
Answers
The conclusion we can draw from the above observation is the given reaction will be proceed faster in the presence of the Manganese oxide as it activation energy of the reaction in the presence of the Manganese oxide is lesser .
The substance that is increases the rate of the reaction without the itself undergoing any of the permanent change chemically or the quantitatively is called the catalyst. The minimum energy in the excess of the normal energy that is the molecules will acquire so to react in the collision with the each other is called the activation energy.
2H₂O₂ ----> H₂O + O₂
The reaction occurs in the presence of the either acetanilide , Ea is 112 kJ/mol or the magnesium oxide ,Ea is 49 kJ/mol. the activation energy of the reaction in the presence of the Manganese oxide is lesser than in the presence of the acetanilide. Therefore, the given reaction will be proceed faster in the presence of the Manganese oxide .
To learn more about activation energy here
https://brainly.com/question/12977580
#SPJ4
Which formula represents a molecular substance? 1. CaO 2. CO 3. Li2O 4. Al2O3
Answers
2. CO- Carbon monoxide
3. Li2O- Lithium oxide
4. Al2O3- Aluminium oxide
linalyl acetate (bergamot oil): C , 73.41 % ; H , 10.29 % ; O , 16.30 %
Express your answer as a chemical formula.
Answers
Empirical formula tells us the relative ratios of different atoms in a compound.
The chemical formula for a compound obtained by composition analysis is always the empirical formula.
How to calculate Empirical formula:
First, try to write the details, like in a tabular formThen, write the given percentage of the elements under themDivide the given % with the atomic mass of the respective elementsAfter getting the values, divide those values with the least value from among them to get a simplest whole numberLinalyl acetate (bergamot oil):
Carbon Hydrogen Oxygen
73.41 % 10.29 % 16.30 %
73.41 / 12 10.29 / 1 16.30 / 16
6.11 10.29 1.01
6.11 / 1.01 10.29 / 1.01 1.01 / 1.01
6 10 1
Empirical formula : C₆H₁₀O
Thus we can conclude that empirical formula of linalyl acetate is C₆H₁₀O
Learn more about Empirical formula at https://brainly.com/question/1603500
#SPJ9
The empirical formula for Linalyl acetate expresses the relative proportions of different atoms in a compound.
What exactly is Linalyl acetate?Linalyl acetate is a phytochemical found in a variety of flowers and spice plants. It is an important component of bergamot and lavender essential oils. It is the linalool acetate ester, and the two are frequently found together.
Linalyl acetate, the primary constituent of lavender oil, is a fragrance chemical commonly found in scented products.Linalyl acetate has very low acute toxicity in mammals; the acute oral LD50 exceeds 13,360 mg/kg, and the inhalation LC50 exceeds 2740 mg/m3. Linalyl acetate causes no or very little irritation in humans. There is no information available about possible eye irritation.Linalyl acetate occurs naturally in organisms such as Xylopia aromatic, Citrus tankan, and others for which data is available.Linalyl acetate is safe as a fragrance material at current concentrations.A food additive is used to improve the flavor or odor of food.A substance that kills or slows the growth of microorganisms like bacteria, viruses, fungi, and protozoans.Hence, the empirical formula describes the relative proportions of different atoms in a compound. The chemical formula for a compound obtained through composition analysis is always the empirical formula.
To learn more about linalyl acetate refer to:
https://brainly.com/question/14983528
#SPJ9
name the structure in the figure in which an electron transport chain is located. describe the main function of the processes that occur in this structure.
Answers
The structure in the figure where an electron transport chain is located is the inner mitochondrial membrane.
The main function of the processes that occur in the inner mitochondrial membrane, specifically in the electron transport chain, is to generate ATP through oxidative phosphorylation.
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. It plays a crucial role in the final stage of cellular respiration, which is the process by which cells extract energy from nutrients.
During oxidative phosphorylation, electrons are transferred through the electron transport chain from energy-rich molecules such as NADH and FADH2.
As electrons pass through the protein complexes, their energy is gradually released, and protons (H+) are pumped across the inner mitochondrial membrane from the mitochondrial matrix to the intermembrane space. This creates an electrochemical gradient.
The main function of this electron transport and proton pumping is to establish a proton motive force.
The gradient created by the electron transport chain drives the ATP synthase enzyme, located in the inner mitochondrial membrane, to produce ATP from ADP and inorganic phosphate. This process is known as chemiosmosis.
Overall, the electron transport chain in the inner mitochondrial membrane plays a crucial role in generating ATP, the energy currency of the cell, by utilizing the energy stored in the electrons derived from the breakdown of nutrients.
To know more about "Mitochondrial membrane" refer here:
https://brainly.com/question/31797295#
#SPJ11
if you mistakenly extract the solution first with naoh (aq), and then with nahco3(aq), what results you will observe and why?
Answers
The NaOH extraction step would remove some acidic components, while the NaHCO3 extraction step may have limited effect if the significant acidic components have already been neutralized.
If you mistakenly extract a solution first with NaOH (aq) and then with NaHCO3 (aq), you would observe the following results:
NaOH Extraction:
When NaOH (aq) is added to the solution, it will react with acidic components present in the solution, such as carboxylic acids, phenols, or acidic functional groups. This reaction results in the formation of water-soluble salts or compounds, which will dissolve in the aqueous NaOH solution. As a result, the acidic components will be removed from the solution.
NaHCO3 Extraction:
When NaHCO3 (aq) is added to the remaining solution from the previous step, it will react with acidic components that were not neutralized by NaOH. NaHCO3 is a weaker base compared to NaOH and is primarily used to extract acidic compounds such as phenols and carboxylic acids. These acidic components will react with NaHCO3 to form water-soluble salts, which will dissolve in the aqueous NaHCO3 solution.
However, if NaOH is mistakenly used first, it is possible that some acidic components in the solution may have already reacted and been removed in the previous step. Therefore, the NaHCO3 extraction step may not yield significant additional changes or observable results.The results of mistakenly extracting the solution first with NaOH (aq) and then with NaHCO3 (aq) would depend on the nature and concentration of the acidic components present in the solution.
Learn more about carboxylic acids visit:
brainly.com/question/4721247
#SPJ11
Question Content Area
How many grams of potassium sulfate would dissolve in 225 g of water to make a saturated solution?
Potassium sulfate has a solubility of 15 g/100 g water at 40°C. A solution is prepared by adding 46 g of potassium sulfate to 225 g of water, carefully heating the solution, and cooling it to 40°C. A homogeneous solution is obtained.
g
Answers
At 40°C 12.25 g potassium sulfate would be precipitated
Saturated solutions are those in which the maximum amount of solute has been dissolved. The solute precipitates out of the solution as more solute is added.
Solubility of potassium sulfate is shown as:
Solubility of potassium sulfate at 40 °C will be 15 g/100 g
It can be understand that at 40 °C 15 g of potassium sulfate would get completely dissolved in 100 of water.
46.0 g of potassium sulfate to 225 g water
Amount of potassium sulfate will get dissolve in 225 g of water at 40 °C will be:
15 g/100g ×225 g = 33.75 g
Amount of potassium sulfate precipitated by the solution can be determined by as follow:
= 39.0 g-33.75 g = 5.25 g
At 40 °C 5.25 g of potassium sulfate will get precipitate out from the solution which means that solution is saturated.
It is given that, the amount of potassium sulfate which is added :
46 g - 33.75 g = 12.25 g.
Therefore, at 40°C 12.25 g potassium sulfate would be precipitated.
To know more about precipitation.
https://brainly.com/question/4703018
#SPJ1
When 23 grams of sodium react with 32 grams of sulfur according to the equation, how many total grams of sodium sulfide should be formed?
Answers
Answer:
78 grams of sodium sulfide should be formed
Explanation:
The balanced chemical equation for the reaction between sodium and sulfur is:
2 Na + S → Na2S
According to the equation, 2 moles of sodium react with 1 mole of sulfur to produce 1 mole of sodium sulfide. The molar mass of sodium is approximately 23 g/mol and the molar mass of sulfur is approximately 32 g/mol.
We need to determine which reactant is limiting and which is in excess in order to calculate the amount of sodium sulfide produced.
Using the given masses, we can calculate the number of moles of each reactant:
moles of sodium = 23 g / 23 g/mol = 1 mol
moles of sulfur = 32 g / 32 g/mol = 1 mol
From this calculation, we can see that both reactants are present in the stoichiometric ratio required by the balanced equation, so neither is limiting.
Therefore, the amount of sodium sulfide formed will be based on the amount of either reactant, which is 1 mole. Using the molar mass of sodium sulfide (78 g/mol), we can calculate the mass of sodium sulfide formed:
mass of Na2S = 1 mol x 78 g/mol = 78 g
Therefore, when 23 grams of sodium react with 32 grams of sulfur, a total of 78 grams of sodium sulfide should be formed.
How many grams of water are needed to absorb 456 J if its temperature goes from 22.7 to 98.3 Celsius?
Answers
The mass of water needed to absorb 456 J is 1.44 g
We'll begin by calculating the change in the temperature of the water.
Initial temperature of water (T₁) = 22.7 °C
Final temperature (T₂) = 98.3 °C
Change in temperature (ΔT) =?ΔT = T₂ – T₁
ΔT = 98.3 – 22.7
ΔT = 75.6 °CFinally, we shall determine the mass of the waterHeat absorbed (Q) = 456 J
Change in temperature (ΔT) = 75.6 °C
Specific heat capacity of water (C) = 4.184 J/gºC
Mass of water (M) =?Q = MCΔT
456 = M × 4.184 × 75.6
456 = M × 316.3104
Divide both side by 316.3104
M = 456 / 316.3104
M = 1.44 gTherefore, the mass of the water is 1.44 g
Learn more: https://brainly.com/question/15563205
Nitric acid + Ammonium chloride →
is it NR? (No reaction)
Answers
Answer:
Yes.
Explanation:
NH4Cl + H2O = NH4+ + HCl (equation 1). Cl- + H2O = H+ Cl- +H2O (equation 2). The chloride (Cl-) first associates with water ( H2O) to form hydrochloric acid (HCl) and the dissociation of HCl produces hydrogen ions (H+).
Plz plz help
Which model shows 5 electrons in the outer shell of the atom?
A.2
B.3
C.4
D.1

Answers
Answer:
D
Explanation:
there are 5 electrons in the outer shell of the first picture
According to electronic configuration, model 1 shows 5 electrons in the outer shell of the atom.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ2
Why are ionic compounds not considered individual molecules?
Answers
Molecules are compounds that make covalent bonds between atom. In a covalent bond, the electrons from each atom invoved are shared between them, sticking the atoms together.
In ionic compounds, the electrons of each atoms are not shared, they are part of either of the ions. The ions sticke together because they are charged with opposite charges, so they attract each other.
So, ionic compounds are not considered individual molecules because they don't make covalent bonds, that is, their atoms don't shared electrons, they attract each other because of their opposite charges.
You have a cell with 100 mM CaCl2 in the ICF (none in the ECF) and 100 mm MgCl2 in the ECF (none in the ICF). After the membrane becomes permeable ONLY to Mg2+, which of the following will occur? There will be net flux of Ca2+ from in to out. There will be a net flux of Mg2+ from out to in and a net flux of Ca2+ from in to out, and no membrane potential will develop. A positive membrane potential will develop due to ion flux through the channels. A negative membrane potential will develop due to ion flux through the channels. Net diffusion of Mg2+ will occur from out to in until the electrical gradient driving force = the concentration grading driving force. Net diffusion of Mg2+ will occur until the concentration of Mg2+ in the ICF = the concentration of Mg2+ in the ECF, and no membrane potential will develop.
Answers
After the membrane becomes permeable only to Mg2+, there will be a net flux of Mg2+ from the extracellular fluid (ECF) to the intracellular fluid (ICF) and a net flux of Ca2+ from the ICF to the ECF. No membrane potential will develop in this scenario.
When the membrane becomes permeable only to Mg2+, Mg2+ ions will move down their concentration gradient from the higher concentration in the ECF to the lower concentration in the ICF. This will result in a net flux of Mg2+ from the ECF to the ICF.
At the same time, Ca2+ ions in the ICF will also move down their concentration gradient from the higher concentration in the ICF to the lower concentration in the ECF. This will lead to a net flux of Ca2+ from the ICF to the ECF.
Since Mg2+ and Ca2+ are both positive ions, their movement across the membrane will not result in the development of a membrane potential. The driving forces for Mg2+ and Ca2+ movement are concentration gradients, not electrical gradients. Therefore, no membrane potential will develop in this situation.
In summary, the net flux of Mg2+ will occur from the ECF to the ICF, the net flux of Ca2+ will occur from the ICF to the ECF, and no membrane potential will be generated.
Learn more about positive ions here :
https://brainly.com/question/14034095
#SPJ11
what is the correct method to dispose of the benzoic acid and phenanthrene produced in this experiment?
Answers
Answer:
The correct method to dispose of the benzoic acid and phenanthrene produced in this experiment is to collect them into a container marked "Hazardous Waste" and dispose of it according to the guidelines of the local waste disposal authority.
Explanation:
Chemical waste must be disposed of carefully to avoid harming the environment or people. Benzoic acid and phenanthrene are two common chemicals that are frequently used in chemistry experiments.
What is benzoic acid?
Benzoic acid is a colorless crystalline solid that is soluble in water and alcohol but insoluble in benzene, toluene, and ether. It has a sweet odor that is similar to that of benzene. Benzoic acid is used as a food preservative and to treat various medical conditions, including urinary tract infections and fungal infections. In the lab, it's often used as a reagent in organic chemistry.
What is phenanthrene?
Phenanthrene is an aromatic hydrocarbon with a formula of C14H10. It's a white crystalline solid that's insoluble in water but soluble in most organic solvents. Phenanthrene is used in the production of dyes, agrochemicals, and pharmaceuticals. In the lab, it is commonly used as a starting material in organic synthesis reactions.
To know more about benzoic acid refer here: https://brainly.com/question/28326761#
#SPJ11
Return to the Projects worksheet. In the range I17:J22, Hwan wants to display the project estimate amounts per state. He can retrieve this information from the PivotTable on the Projects by State worksheet. Display the estimates per state as follows:
a. In cell J18, insert a formula using the GETPIVOTDATA function to display the total estimate amount for projects in Montana from cell G5 on the Projects by State worksheet.
b. In cell J19, insert a formula using the GETPIVOTDATA function to display the total estimate amount for projects in North Dakota from cell G10 on the Projects by State worksheet.
c. In cell J20, insert a formula using the GETPIVOTDATA function to display the total estimate amount for projects in Nebraska from cell G15 on the Projects by State worksheet.
d. In cell J21, insert a formula using the GETPIVOTDATA function to display the total estimate amount for projects in South Dakota from cell G21 on the Projects by State worksheet.
e. In cell J22, insert a formula using the GETPIVOTDATA function to display the total estimate amount for projects in Wyoming from cell G26 on the Projects by State worksheet.
Answers
This fοrmula retrieves the tοtal estimate amοunt fοr prοjects in Wyοming frοm cell G26 οn the Prοjects by State wοrksheet.
What is Excel in simple wοrds?Excel is a spreadsheet prοgram frοm Micrοsοft and a cοmpοnent οf its Office prοduct grοup fοr business applicatiοns. Micrοsοft Excel enables users tο fοrmat, οrganize and calculate data in a spreadsheet.
To display the estimate amounts per state using the GETPIVOTDATA function in Excel, follow the instructions below:
a. In cell J18, enter the following formula:
swift
=GETPIVOTDATA("Estimate",$A$5,"State","Montana")
This formula retrieves the total estimate amount for projects in Montana from cell G5 on the Projects by State worksheet.
b. In cell J19, enter the following formula:
swift
=GETPIVOTDATA("Estimate",$A$5,"State","North Dakota")
This formula retrieves the total estimate amount for projects in North Dakota from cell G10 on the Projects by State worksheet.
c. In cell J20, enter the following formula:
swift
=GETPIVOTDATA("Estimate",$A$5,"State","Nebraska")
This formula retrieves the total estimate amount for projects in Nebraska from cell G15 on the Projects by State worksheet.
d. In cell J21, enter the following formula:
swift
=GETPIVOTDATA("Estimate",$A$5,"State","South Dakota")
This formula retrieves the total estimate amount for projects in South Dakota from cell G21 on the Projects by State worksheet.
e. In cell J22, enter the following formula:
swift
=GETPIVOTDATA("Estimate",$A$5,"State","Wyoming")
This formula retrieves the total estimate amount for projects in Wyoming from cell G26 on the Projects by State worksheet.
Learn more about Excel
https://brainly.com/question/3441128
#SPJ4
what is the happens in electrolysis if the electrolyte solidifies?
Answers
Answer: For example, if electricity is passed through molten lead bromide, the lead bromide is broken down to form lead and bromine. This is what happens during electrolysis: Positively charged ions move to the negative electrode during electrolysis. ... Negatively charged ions move to the positive electrode during electrolysis.
Explanation:
hope this helps you find what your looking for
11. It is proposed to build a plant to produce 170,000 t·y−1 of a commodity chemical. A study of the supply and demand projections for the product indicates that current installed capacity in the industry is 6.8 × 106 t·y−1, whereas total production is running at 5.0 × 106 t·y−1. Maximum plant utilization is thought to be around 90%. If the demand for the product is expected to grow at 8% per year, and it will take 3 years to commission a new plant from the start of a project, what do you conclude about the prospect for the proposed project?
Answers
To analyze the prospect for the proposed project, let's consider the given information and calculate the projected demand and supply over the next few years:
Current installed capacity: 6.8 × 10^6 t·y^(-1)
Total production: 5.0 × 10^6 t·y^(-1)
Maximum plant utilization: 90%
Desired production: 170,000 t·y^(-1)
Demand growth rate: 8% per year
Project commissioning time: 3 years
Year 1:
Demand = Total production + (Demand growth rate × Total production)
= 5.0 × 10^6 + (8/100 × 5.0 × 10^6)
= 5.4 × 10^6 t·y^(-1)
Year 2:
Demand = Year 1 demand + (Demand growth rate × Year 1 demand)
= 5.4 × 10^6 + (8/100 × 5.4 × 10^6)
= 5.83 × 10^6 t·y^(-1)
Year 3:
Demand = Year 2 demand + (Demand growth rate × Year 2 demand)
= 5.83 × 10^6 + (8/100 × 5.83 × 10^6)
= 6.29 × 10^6 t·y^(-1)
Next, we need to determine the maximum production capacity considering a 90% plant utilization rate:
Maximum production capacity = Current installed capacity × Plant utilization rate
= 6.8 × 10^6 × 0.9
= 6.12 × 10^6 t·y^(-1)
Based on the projected demand and maximum production capacity, we can evaluate the prospects for the proposed project:
Year 1: Demand (5.4 × 10^6 t·y^(-1)) < Maximum production capacity (6.12 × 10^6 t·y^(-1))
Year 2: Demand (5.83 × 10^6 t·y^(-1)) < Maximum production capacity (6.12 × 10^6 t·y^(-1))
Year 3: Demand (6.29 × 10^6 t·y^(-1)) > Maximum production capacity (6.12 × 10^6 t·y^(-1))
It is important to note that other factors such as market competitiveness, cost analysis, and financial viability should also be considered before making a final conclusion about the prospects of the proposed project.
To learn more about capacity
https://brainly.com/question/29792498
#SPJ11
When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of sodium...
A) Loses an electron
B) Loses a proton
C) Becomes an ion with a larger radius than the atom of sodium
D) Gains an electron
Answers
When an atom of chlorine forms an ionic bond with an atom of sodium, the
atom of sodium loses an electron.
What is an Ionic bond?This is also known as electrovalent bond and it is the bond which occurs
as a result of elements gaining or losing electrons in a reaction.
An atom of chlorine forming an ionic bond with an atom of sodium,means
the atom of sodium will have to lose an electron in other to achieve a
stable octet configuration.
Read more about Ionic bonding here https://brainly.com/question/13526463
What type of energy transformation occurs during photosynthesis?
Answers
Answer: Photosynthesis is the process by which energy is converted to chemical energy in plant cells. In cellular respiration plants use the chemical energy stored during photosynthesis in basic life processes. During both photosynthesis and cellular respiration, energy is converted.
Explanation: