Joel reacted 0.9845 g of iron filings with an excess of copper sulphate solution. When the reaction was complete the solution had changed from blue to green and a copper deposit weighing 1.024 g had formed in the solution. In this reaction, metallic iron is converted into ferrous ion (Fe2+) and cupric ion (Cu2+) is converted into metallic copper.
a) Calculate the number of moles of iron used and the number of moles of copper
formed.
b) Calculate the theoretical mass of copper that was expected to be produced and
hence deter,mine the percentage yield of Joel’s experiment.
c) How many molecules of iron are present in 0.9845 g of iron fillings?
Fiona prepared a solution by dissolving 1.35 g of silver nitrate in a 250 mL volumetric flask and diluting it up to the volume.
d) Calculate the molarity of the silver nitrate solution.
e) What volume of this solution is required to prepare 100 mL of 0.01 M of silver nitrate solution?
Answers
From the stoichiometry of the reaction, the percentage yiled of the copper is 89.6%
What is reaction equation?The reaction equation here is given as; Fe(s) + CuSO4 (aq) ----->FeSO4(aq) + Cu(s)
Number of moles of Fe = 0.9845 g/56 g/mol = 0.018 moles. Since the reaction is 1:1, this is also the number of moles of Cu formed.
Theoretical yiled of Cu = 0.018 moles * 63.5 g/mol = 1.143 g
Percentage yiled = 1.024 g/1.143 g * 100/1 = 89.6%
If 1 mole of Fe contains 6.02 * 10^23 molecules
0.018 moles of Fe contains 0.018 moles * 6.02 * 10^23 molecules/1 mole = 1.1 * 10^22 molecules.
Number of moles of AgNO3 = 1.35 g/170 g/mol = 0.0079 moles
Molarity of the solution = 0.0079 moles/250 * 10^-3 L = 0.0032 M
Hence; 0.0032 M * V1 = 100 mL * 0.01 M
V1 = 312.5 mL
Learn more about stoichiometry: https://brainly.com/question/9743981
#SPJ1
Related Questions
what are 2 ways that all hydrocarbons are alike?
Answers
Answer:
Composition: All hydrocarbons are made up of only two types of atoms: carbon and hydrogen. They are like building blocks that contain carbon and hydrogen stuck together.
Organic Nature: Hydrocarbons are special because they are part of a group of compounds that come from living things or things that were once alive. They have carbon and hydrogen in them, which is what makes them different from other types of compounds.
Explanation:
How many grams of CaSO4 form when
50.0 mL of 0.20 M Ca(NO3)2 react with
excess potassium sulfate solution?
Ca(NO3)2 + K2SO4 → CaSO4 + 2KNO3
-
?] g CaSO4
Answers
Answer:
1.36g CaSO4 (FIXED THIS)Explanation:
.01 mol CaSO4 is the conversion factor between these values and now you just need to multiply it by the molar mass of .01 x 136.14=1.36g CaSO4
Apologies for having the wrong answer before this, it's been a long day.
convert 8.42x10^8 mol/(kg*m^2) to mol/(g*cm^2)
Answers
Answer:
gguhg
Explanation:
no te es caso drama me están muy una las y y que las te
1 kg = 1000 g
1 m = 100 cm
Using these equations, 8.42x10^8 mol/(kgm^2) can be converted to 8.42x10^11 mol/(gcm^2).
1. Which statement is true about the relationship between chromosomes, genes and
traits?
A. Genes are found within traits, and their codes are used to make chromosomes.
B. Chromosomes are found within genes, and their codes are used to make traits.
C. Genes are found within chromosomes, and their codes are used to make traits.
D. One gene is found on every chromosome and they are used to make traits.
Answers
Answer:
the answer is C.Explanation:
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
PLEASE!!!
Calculate the mass of 6.022×10236.022×1023 molecule of NH4cl
Answers
Answer:
53.5gram.
Explanation
Explanation: number of moles = number of molecule ÷ avogadro's number. => moles = 6.022 × 10²³ ÷ 6.022 × 10²³ = 1 mole. mass of 1 mole of NH4Cl = 1 × molar mass.
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
Can a chemical reaction be both exothermic and endothermic? Explain.
Answers
Answer:
It cannot be both, because the heat released or absorbed is also equal to the difference in total heat content of the reactants and products. So, it cannot be both.
Answer:
B
Explanation:
A sample of an unknown compound is vaporized at 150.°C . The gas produced has a volume of 960.mL at a pressure of 1.00atm , and it weighs 0.941g . Assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. Round your answer to 3 significant digits.
Answers
The molar mass of the unknown compound that vaporizes at 150 °C is 34.81 g/mol
What is the molar mass of a compound?The molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the number of moles in that sample. Its unit of measurement is grams per mole (g/mol) although in the International System of Measurements it should be kilograms per mole (kg/mol).
This is a property closely related to the relative molar mass of a compound and to the standard atomic masses of the constituent elements.
Determination of the molar mass of the unknown gasTo determine the molar mass of the gas, the ideal gas equation was used: PV = nrt, in which
P = Pressure
V = Volume
n = number of moles
R = Gas constant
t = temperature
But previously the moles of the gas are calculated
Calculate the moles of the unknown compoundData
t = 150°C = 423.15K
V = 960ml = 0.96L
P = 1.00 atm
R = 0.082 L Atm / mol °K .
n = ?
PV = nrt
n = PV/rt
n=1. 0.96 / 0.082. 423.15
n = 0.96 / 34.7
n = 0.027 mole
Calculation of the molar mass of the unknown compoundM = m/n
M = 0.94 g/ 0.027 mol
M = 34.81 g/mol
Learn more about molar mass at https://brainly.com/question/13152455
#SPJ4

A fire women dropped a person onto the safety net.Right before the person hit the net he had a velocity of 11.2m/s and 1800 J of kinetic energy. What was the mass of the person?
Answers
Explanation:
kinetic energy = ½mv²
1800 = ½ × m × 11.2²
1800 = ½ × m × 125.44
3600 = 125.44m
m = 3600/125.44
m = 28.7 Kg
true or false When energy and matter are paired with light, they are one in the same.
Answers
Answer:
when energy and matter are paired together ,they are truly one in same
The statement "When energy and matter are paired with light, they are one and the same" is definitely true.
What do you mean by Matter?The Matter may be defined as a type of substance that is significantly made up of numerous types of particles that occupy the physical space and possess inertia. Everything that is present in the surroundings represents an example of matter.
According to the context of this question, energy and matter togetherly constitute the principle of the same component. This is because energy is a property that matter possesses to a large extent.
It may be thought the same amount of matter can have a different amount of energy and when they both get paired with light, they are one and the same.
Therefore, the statement "When energy and matter are paired with light, they are one and the same" is definitely true.
To learn more about Matter, refer to the link:
https://brainly.com/question/3998772
#SPJ2
2.50 g of As2O3 are titrated with 38.5 mL of KMnO4 to reach the end point.
5As2O3(s)+4MnO−4(aq)+9H2O(l)+12H+(aq)⟶10H3AsO4(aq)+4Mn2+(aq)
Calculate the concentration of the KMnO4 solution.
Answers
50 g of As\(_2\)O\(_3\) are titrated with 38.5 mL of KMnO\(_4\) to reach the end point. 0.26M is the concentration of the KMnO\(_4\) solution.
Concentration in chemistry refers to the quantity of a material in a certain area. The ratio of the solute within a solution to the solvent or whole solution is another way to define concentration. In order to express concentration, mass in unit volume is typically used.
The solute concentration can, however, alternatively be stated in moles or volumetric units. Concentration may be expressed as per unit mass rather than volume.
5As\(_2\)O\(_3\)(s)+4MnO\(_4\)⁻(aq)+9H\(_2\)O(l)+12H⁺(aq)⟶10H\(_3\)AsO\(_4\)(aq)+4Mn\(_2\)⁺(aq)
the stoichiometry ratio between As\(_2\)O\(_3\) and MnO\(_4\)⁻ is 5:4
0.0126 moles of As\(_2\)O\(_3\) will react with 4/5×0.0126 moles = 0.01008moles
0.01008moles of MnO\(_4\)⁻ is present in 38mL
concentration of KMnO\(_4\)= moles×volume
= 0.010/38×1000
=0.26M
To know more about Concentration, here:
https://brainly.com/question/10725862
#SPJ1
What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
Which statement best illustrates how mixtures and pure substances are different?
Mixtures have color; pure substances are colorless.
Mixtures have various odors; pure substances are odorless.
Mixtures are found on the periodic table; pure substances are not.
Mixtures are physically combined; pure substances are chemically combined.
Answers
Answer:
Mixtures are physically combined and pure substances are chemically combined.How many grams of a 26.9% sugar solution contain 49.0 g of sugar?
Answers
Answer:
182.156g
Explanation:
grams = 49/.269 = 182.156g needed
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
How much did asbestos exposure decrease during the year 1982 and 1983

Answers
The asbestos exposure during the years 1982 and 1983 was 2.5 fibers per cubic centimeter and 0.8 fibers per cubic centimeter respectively. So asbestos exposure decreased by 1.7 fibers per cubic centimeter during the year 1983.
Breathlessness Persistent, dry cough Chest pain or tightness Lack of a dry, crackling sound in the lungs when you breathe in Wider and rounder fingers and toes are some of the symptoms of asbestos exposure.
The largest group of people exposed to asbestos is those working in the construction industry. Historically, asbestos was also used by pipe fitters and shipyard workers. In addition, asbestos was used by military personnel, auto mechanics, and many other occupations.
To learn more about asbestos, refer to the link:
https://brainly.com/question/8853025
#SPJ1
I NEED HELP ASAP!!!
Which answer below correctly identifies the type of change and the explanation when potassium iodide and lead nitrate are mixed?
physical change because the observation of a solid forming is evidence of a state change, which is reversible
physical change because even though the mixture had a color that was different from either of the two solids alone, each solid's physical properties remained exactly the same
chemical change because two substances were mixed, which always results in the formation of a
new substance
chemical change because both a color change and a solid formation were observed, which provide strong evidence of a new substance
Answers
Answer:
The correct answer is chemical change because both a color change and a solid formation were observed, which provide strong evidence of a new substance.
A physical change is a change in the state of a substance, such as a change from a solid to a liquid or a change from a liquid to a gas. A chemical change is a change in the composition of a substance, such as the formation of a new substance.
When potassium iodide and lead nitrate are mixed, a yellow precipitate forms. This precipitate is a new substance that was not present before the two substances were mixed. Therefore, the change that occurs when potassium iodide and lead nitrate are mixed is a chemical change.
The other answers are incorrect.
* Answer 1 is incorrect because the observation of a solid forming is not evidence of a state change. A state change is a change in the physical state of a substance, such as a change from a solid to a liquid or a change from a liquid to a gas. The formation of a precipitate is not a state change, but rather a chemical change.
* Answer 2 is incorrect because the color change of the mixture is evidence of a chemical change. When two substances are mixed and a new substance is formed, the new substance may have a different color than the original substances.
* Answer 3 is incorrect because the statement "two substances were mixed, which always results in the formation of a new substance" is not always true. For example, if you mix two different types of liquids, you may not get a new substance. Instead, you may just get a mixture of the two liquids.
The reaction between potassium iodide and lead nitrate results in a chemical change because a color change and solid formation, indicative of a new substance, are observed.
Explanation:When potassium iodide and lead nitrate are mixed, there is a chemical change that takes place. This is because both a color change and a solid formation were observed, which provide strong evidence of a new substance. In this reaction, two new compounds are formed - lead iodide and potassium nitrate - which is a clear indication of a chemical change. This process is not easily reversible, further supporting it being a chemical change.
Learn more about Chemical Change here:https://brainly.com/question/31633022
#SPJ2
g Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a tank with of ammonia gas and of oxygen gas, and when the mixture has come to equilibrium measures the amount of water vapor to be . Calculate the concentration equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to significant digits.
Answers
\(\text{Ammonia has been studied as an alternative "clean" fuel for internal combustion}\)
\(\text{engines, since its reaction with oxygen produces only nitrogen and water vapor,}\)
\(\text{and in the liquid form it is easily transported. An industrial chemist studying this}\)
\(\text{reaction fills a} \ \mathbf{100 \ L }\ \text{tank with} \ \mathbf{8.6 \ mol} \ \text{of ammonia gas and} \ \mathbf{28 \ mol} \ \ \text{of oxygen gas, }\)
\(\text{to be} \ \mathbf{2.6\ mol} \ .\ \text{Calculate the concentration equilibrium constant for the combustion of}\)
\(\text{ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.}\)
Answer:
Explanation:
From the correct question above:
The reaction can be represented as:
\(\mathbf{4 NH_3_{(g)}+ 3O_{2(g)} \iff 2N_{2(g)}+ 6H_2O_{(g)} }\)
From the above reaction; the ICE table can be represented as:
\(\mathbf{4 NH_3_{(g)}+ 3O_{2(g)} \iff 2N_{2(g)}+ 6H_2O_{(g)} }\)
I (mol/L) 0.086 0.28 0 0
C -4x -3x +2x +6x
E 0.086 - 4x 0.28 - 3x +2x +6x
At equilibrium;
The water vapor = \(\dfrac{2.6 \ mol}{100 \ L} = 6x\)
\(x = \dfrac{2.6}{100} \times \dfrac{1}{6}\)
\(x = 0.00433\)
\(\text{equilibrium constant} ({k_c}) = \dfrac{ [N_2]^2 [H_2O]^6 }{ [[NH_3]^4] [O_2]^3 }\)
\(\implies \dfrac{(2x)^2 (6x)^6}{(0.086-4x)^4\times (0.28-3x)^3} \\ \\\)
Replacing the value of x, we have:
\(K_c = \dfrac{4 \times 46,656 \times x^8}{(0.086-4x)^4\times (0.28 -3x)^3} \\ \\ K_c = \dfrac{4 \times 46656 \times (0.00433)^8}{(0.06868)^4(0.26701)^3} \\ \\ K_c = \mathbf{5.4446 \times 10^{-8}}\)
\(K_c = \mathbf{5.5 \times 10^{-8} \ to \ 2 \ significant \ figures}\)
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
what is the photoelctric effect?
Answers
Explanation:
It is the emission of electron from a metal under the effect of light is known as photo electric effect
I hope this imformation help full for you
Help me out
On another planet, the isotopes of titanium have the given natural abundances.

Answers
The average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u). The average atomic mass may vary depending on the specific isotopic composition of titanium found on different celestial bodies or regions.
To calculate the average atomic mass of titanium on the given planet, we need to consider the natural abundances and masses of each isotope of titanium.
The average atomic mass is calculated by multiplying the natural abundance of each isotope by its respective mass and summing them up.
Let's perform the calculation step by step:
Step 1: Multiply the abundance of each isotope by its mass:
(73.700% * 45.95263 u) + (15.000% * 47.94795 u) + (11.300% * 49.94479 u)
Step 2: Calculate the individual contributions from each isotope:
= (0.737 * 45.95263) + (0.150 * 47.94795) + (0.113 * 49.94479)
Step 3: Add up the individual contributions:
= 33.84765431 + 7.1921925 + 5.64179347
Step 4: Sum up the contributions:
= 46.68164 u
Therefore, the average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u).
It's important to note that the calculation assumes the provided natural abundances are accurate and representative of the titanium isotopes on that planet.
for more questions on atomic mass
https://brainly.com/question/30390726
#SPJ8
Mg + HCl -> MgCl2 + H2
When the equation is balanced what should the coefficient for magnesium chloride be

Answers
Explanation:
hope it helps you
have a good day please mark me as brain list ☺️ sorry for picture

Under what conditions will bacteria thrive? Explain what you can do to alter these conditions.
Answers
Bacteria thrive under specific conditions that provide them with the necessary resources for growth and reproduction. These conditions can vary depending on the type of bacteria, but there are some general factors that contribute to their proliferation.
Temperature: Bacteria have preferred temperature ranges for growth. For example, mesophilic bacteria thrive at moderate temperatures (20-45°C), while psychrophilic bacteria prefer colder environments (0-20°C). To alter these conditions, adjusting the temperature either up or down within the desired range can inhibit or promote bacterial growth.
pH Levels: Bacteria have different pH requirements, ranging from acidophilic (acid-loving) to alkaliphilic (alkali-loving). Modifying the pH level by adding acids or bases can create an environment unfavorable for bacterial growth.
Moisture: Most bacteria require moisture for survival and growth. Altering the moisture content can impact bacterial proliferation. Reducing moisture through drying or increasing it through moisture control methods can affect bacterial growth.
Nutrient availability: Bacteria need essential nutrients such as carbon, nitrogen, phosphorus, and trace elements. Adjusting the nutrient availability can be done by modifying the composition of the growth medium, limiting access to specific nutrients, or utilizing antimicrobial agents.
Oxygen requirements: Bacteria can be classified as aerobic (requiring oxygen), anaerobic (thriving in the absence of oxygen), or facultative (able to adapt to both conditions). Controlling oxygen levels by adjusting aeration or creating anaerobic conditions can alter bacterial growth.
To alter these conditions, one can employ various techniques such as adjusting temperature, pH, moisture, nutrient availability, and oxygen levels to inhibit or promote bacterial growth. This can be achieved through environmental modifications, using antimicrobial substances, implementing sterilization techniques, or employing chemical treatments.
For such more questions on proliferation
https://brainly.com/question/14130535
#SPJ11
1 moles of a monoatomic gas increases its volume from 1 to 3 while the pressure also increases from 250 to 1000
Answers
1 moles of a monoatomic gas increases its volume from 1 to 3 while the pressure also increases from 250 to 1000. So, the temperature of the gas increased from 30.1 K to 120.3 K.
If the temperature and the number of moles of the gas remain constant, the pressure and volume of the gas are inversely proportional to each other. This relationship is described by the ideal gas law:
PV = nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the universal gas constant, and T is the temperature of the gas. In this case, you have given that the number of moles of the gas (n) is 1 mole, the temperature (T) is constant, and the pressure (P) and volume (V) both change. We can use the ideal gas law to solve for the final temperature of the gas.
First, we can rearrange the ideal gas law to solve for T:
T = (PV)/(nR)
We can then use the initial and final values of P and V to calculate the initial and final temperatures of the gas.
Initial temperature: T1 = (250 * 1)/(1 * 8.31) = 30.1 K
Final temperature: T2 = (1000 * 3)/(1 * 8.31) = 120.3 K
Therefore, the temperature of the monoatomic gas increased from 30.1 K to 120.3 K as the volume increased from 1 to 3 while the pressure increased from 250 to 1000.
To know more about monoatomic gas please refer: https://brainly.com/question/29746488
#SPJ4
Question- 1 moles of a monoatomic gas increases its volume from 1 to 3 while the pressure also increases from 250 to 1000. What is the change in temperature of gas is?
What is the solubility (in M) of PbCl2 in a 0.15 M solution of HCl? The Ksp of PbCl2 is 1.6 x 10-5.
Answers
7.11 × 10⁻⁴ is the solubility (in M) of PbCl\(_2\) in a 0.15 M solution of HCl. The Ksp of PbCl\(_2\) is 1.6 x 10⁻⁵.
The capability of a material, the solute, to combine with another material, the solvent, is known as solubility in chemistry. Insolubility, or the solute's inability to create such a solution, is the opposite attribute.
The amount of each of the solute within a saturated solution—a solution whereby no more solute is able to be dissolved—is typically used to gauge the degree of a substance's solubility in a particular solvent.
PbCl\(_2\) ⇌ Pb\(_2\)⁺(x) + 2Cl⁻ (2x)
HCl ⇌H⁺ (0.15M) + Cl⁻ (0.15M)
Ksp = {Pb\(_2\)⁺} {Cl⁻}²
Ksp = {x} {2x+ 0.15}²
2x<0.15
Ksp = {x} {0.15}²
x = 7.11 × 10⁻⁴
To know more about solubility, here:
https://brainly.com/question/29661360
#SPJ1
18 Base your answers to the questions on the
information below and on your knowledge of
chemistry.
An operating voltaic cell has
magnesium and silver electrodes. The
cell and the ionic equation representing
the reaction that occurs in the cell are
shown below.
Wire
Mg(s)
electrode
Voltaic Cell
Mg" (aq)
V
Voltmeter
Salt
bridge
Ag (aq)
Ag(s)
electrode
Mg(s) + 2Ag (aq) + Mg(aq) + 2Ag(s)
State the purpose of the salt bridge in this cell.

Answers
The cell's reaction is not disrupted, and the electrons continue to flow between the two half-cells through the wire to produce electricity.
In a voltaic cell, a salt bridge is utilized to maintain the electrical neutrality of the half-cells. A salt bridge is an ionic conductor that allows the movement of cations and anions between the two half-cells without allowing any mixing of the electrolytes.
Its primary function is to prevent the buildup of electric charges on the electrodes, which would result in a sudden stop of the reaction in the cell.What is a salt bridge-A salt bridge is an electrolytic channel that connects the oxidation and reduction half-cells of a voltaic cell to allow ions to flow between them.
The salt bridge is essential to maintain the electrical neutrality of the half-cells and maintain the cell's electrical energy. What is the purpose of the salt bridge in this cell?In the given cell, the salt bridge purpose is to keep the electrical neutrality of the half-cells.
The salt bridge ensures that the cell's two half-cells maintain their neutrality by providing a flow of ions from one half-cell to the other. As a result, the cell's reaction is not disrupted, and the electrons continue to flow between the two half-cells through the wire to produce electricity.
for such more questions on reaction
https://brainly.com/question/26084288
#SPJ8
Which formula is the mathematical representation of Charles’s law?P1V1 = V2/T2P1V1 = P2V2V1/T1 = V2/T2P1/T1 = P2/T2
Answers
Answer:
V1/T1 = V2/T2.
Explanation:
Let's remember Charles's Law: Charles's Law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant.
Wher
Mathematically, this can be described like this:
\(\frac{V}{T}=k.\)We can use Charles's Law to compare changing conditions of gases, like this:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2}.\)Where V is volume, T is the temperature, subindex 1 indicates the initial conditions, and subindex 2 indicates the final conditions.
The answer would be V1/T1 = V2/T2.
How many grams are in 23 moles of Magnesium nitride?
Answers
Answer:2523.75 g
Explanation:
(25 mols)* (100.95g/1 mol)= 2523.75g magnesium nitride
- molar mass of magnesium nitride is 100.95 g/mol
Compare the average motion of the particles in the 3 containers of water
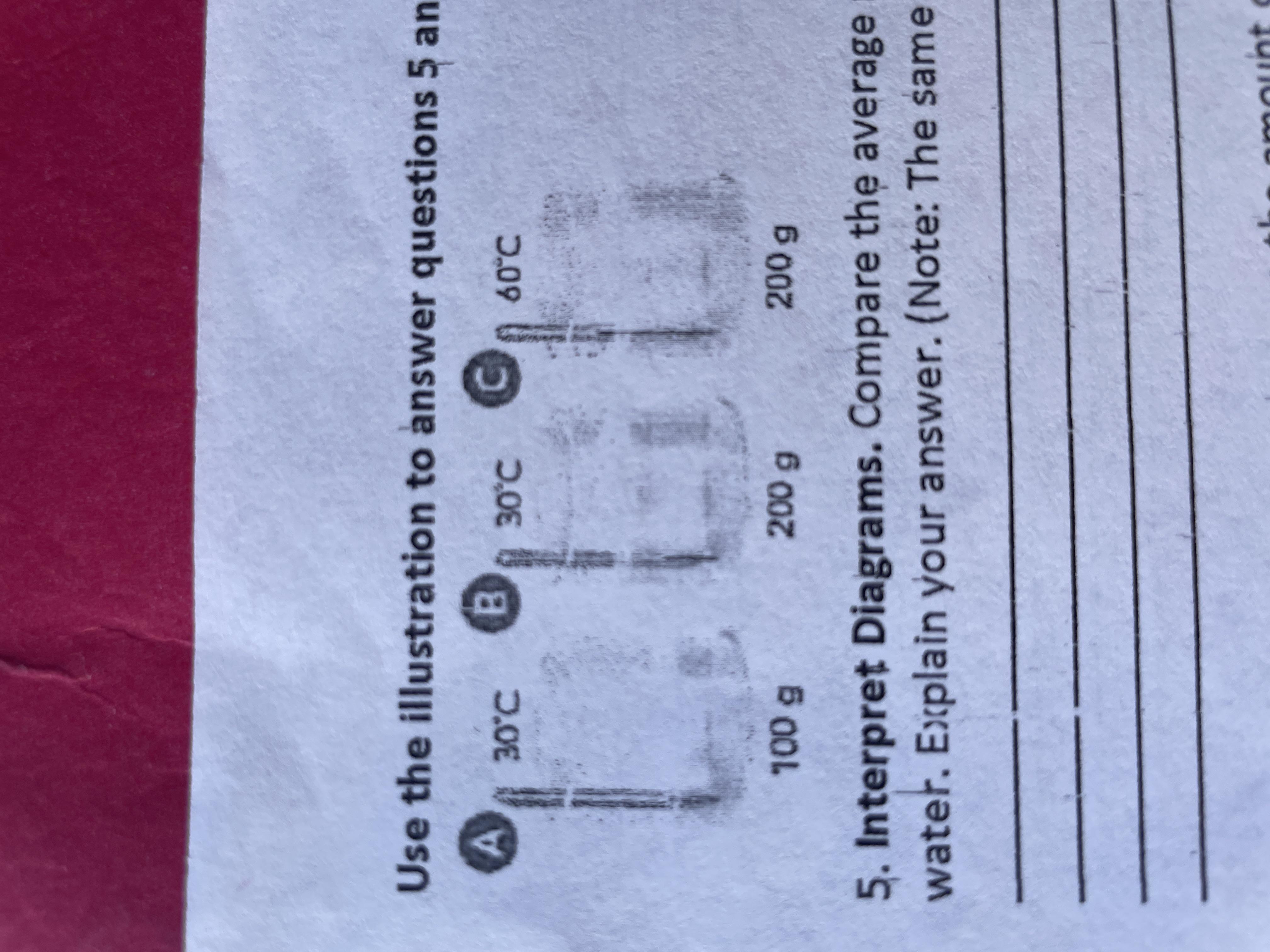
Answers
Answer:
c>b=a
Explanation:
It is important to note that mass does not affect the average motion/energy per molecule, but temperature does. the higher the temperature the faster the particles are. A has the same temperature as B, so they have the same amount of motion. C is warmer than A and B, so the average motion of the particles in beaker C is the largest