List the following particles in size order, from biggest to smallest?
A Molecules, electrons, atoms incorrect answer
B Atoms, molecules, electrons incorrect answer
C Electrons, atoms, molecules incorrect answer
D Molecules, atoms, electrons
Answers
Answer:
D. Molecules, atoms, electrons.
Explanation:
I did a quiz on this and got it right
Answer:
D. Molecules, atoms, electrons.
Explanation:
i did the test trust i got it right
Related Questions
Which property of water allows it to act as a transport medium?
(a) adhesion
(b) the high heat of evaporation
(c) high heat capacity
(d) water is solvent
(e) the frozen form is less dense than the liquid form.
Answers
The property of water that allows it to act as a transport medium will be water is a solvent. Option D is correct.
Water will be often referred to as the "universal solvent" because it has the ability to dissolve a wide range variety of substances. This property is due to the polar nature of the water molecules. Water molecules have a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen atom, creating a polar molecule.
When substances dissolve in water, the polar water molecules surround the solute particles, breaking the ionic or molecular bonds that hold the solute together. This allows the solute to be transported and dispersed throughout the water, making water an effective medium for transporting dissolved substances.
Adhesion refers to the ability of water to stick to other surfaces, while the high heat of evaporation and high heat capacity refer to water's ability to absorb and retain heat. The property mentioned in option, the frozen form of water being less dense than the liquid form (known as the expansion of water upon freezing), is related to its unique crystal lattice structure and not directly related to acting as a transport medium.
Hence, D. is the correct option.
To know more about universal solvent here
https://brainly.com/question/30641446
#SPJ4
the non-metal elements selenium has six electrons in its outer orbit what atoms of this element form positively charged or negatively charged ions
Answers
Answer:
positively charged -protons
Negatively charged-electrons
A scientist dissolves 10 g of NaOH into 0.275 L of water. What is the
molarity of the solution?*
0.3636 M
0.9091 M
O 0.2750 M
0.8956 M
Please help I’m being timed
Answers
When you get home, you smell cookies baking. You respond by walking toward the kitchen. Many signals move through your
nervous system in this process. Which component is involved first? (1 point)
O axons in your motor neurons
axons in your sensory neurons
Odendrites in your motor neurons
O dendrites in your sensory neurons
Answers
Answer:
dendrites in your sensory neurons
Explanation:
Sensory neurons are first activated, then the stimulus is transmitted horizontally between them, spreading perception through the olfactory bulb that captures the smell of a baked cookie.
After this, the axons are activated to transmit to the motor neurons that will carry out the action of walking towards the kitchen.
Step 7: Put the Metal in the Water and Measure
Temperature Changes (Aluminum)
The temperature at 300 seconds (5 minutes) is the
final temperature of both the metal and the water.
Record it to the nearest 0.1°C in the data table.
27
26
25
24
23
05:00
20
Continue (60s more)
www.w
250 mL
223
200
150
100
50
(1-0)
Initial temperature of metal = 100
°C
Initial temperature of water = 22.4
°C
Final temperature of both = 27.1
°C
COMPLETE
Subtract to find the temperature changes
for the water and the metal.
AT (water) =
1°C
AT (metal) =
DONE✔
==
°C
Answers
Change in temperature of the metal, ΔT₁ = 82.9°C
Change in temperature of water, ΔT₂ = 4.7°C
What is temperature?Temperature is a measure of the average kinetic energy of the molecules in a substance.
It is defined as the degree of hotness or coldness of a body.
Temperature changes occur when heat is added or removed from a substance.
To determine the temperature change in water and the meatal when the heated aluminum was placed in water, the following calculations are done:
Initial temperature of metal = 100°C
Initial temperature of water = 22.4°C
Final temperature of both = 27.1°C
Change in temperature of the metal, ΔT₁ = 100 - 27.1
ΔT₁ = 82.9°C
Change in temperature of water, ΔT₂ = 27.1 - 22.4°C
ΔT₂ = 4.7°C
In conclusion, the change in temperature is determined by finding the difference in the temperatures.
Learn more about temperature change at: https://brainly.com/question/24719561
#PSJ1
Answer:
100
22.4
27.1
4.7
72.9
Explanation:
It just is, trust me
Write a balanced molecular equation describing each of the following chemical reactions:
Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
Answers
Answer: CaCO3 = CaO + CO2
Explanation:
CaCO3 = CaO + CO2
This is balanced already.
Elem Reactant Products
Ca 1 1
C 1 1
O 3 3
Study the Lewis dot diagram for nitrogen. Explain what is correct and what is incorrect about the diagram. Use details to support your answer.

Answers
Answer:
The Lewis dot diagram is supposed to have dots on each side. What's incorrect is that there isn't a dot on the bottom, only the left and right side and the top. What's correct about this is that there are 5 outer valence electrons, and they correctly put 5 dots, even though they're in the wrong place.
Explanation:
The given Lewis dot diagram of nitrogen is correct for the number of valence electrons for nitrogen. However, they are not correctly placed because two of them are lone pairs and only these two are shown as paired. Remaining three electrons have to be represented as single electrons.
What is Lewis dot structure?Lewis dot structure of an element represent its valence electrons shown as dots around the atom. The lone pair of electrons are shown as paired dots and other electrons as single dots.
For compounds, a Lewis diagram shows the bonded and non bonded electrons between bonded atoms. Nitrogen is 7th element and it contains 7 electrons in which 5 are valence electrons.
Nitrogen contains one lone pair of electrons. They have to be shown as paired dots in the Lewis structure. In the given diagram there are two pairs of electrons which is incorrect. Three electrons have to be shown as single dots.
To find more on Lewis dots, refer here:
https://brainly.com/question/20300458
#SPJ2
when 2.33 moles of hydrochloric acid reacts completely how many grams of zinc chloride will be produced?
Answers
Assuming excess zinc, 2.33 moles of hydrochloric acid would produce 645.13 grams of zinc chloride.
What is the balanced chemical equation for the reaction between hydrochloric acid and zinc?The balanced chemical equation for the reaction between hydrochloric acid and zinc is: Zn + 2HCl → ZnCl2 + H2.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and zinc (Zn) is:
Zn + 2HCl → ZnCl2 + H2
This equation tells us that 1 mole of zinc reacts with 2 moles of hydrochloric acid to produce 1 mole of zinc chloride and 1 mole of hydrogen gas. Therefore, to determine how much zinc chloride is produced when 2.33 moles of hydrochloric acid reacts completely, we need to use stoichiometry.
First, we need to determine how many moles of zinc chloride would be produced from 2.33 moles of hydrochloric acid. From the balanced equation, we know that the ratio of hydrochloric acid to zinc chloride is 2:1. Therefore, the number of moles of zinc chloride produced would be half the number of moles of hydrochloric acid used:
2.33 moles HCl x (1 mole ZnCl2 / 2 moles HCl) = 1.165 moles ZnCl2
Next, we need to convert moles of zinc chloride to grams. The molar mass of zinc chloride is the sum of the atomic masses of one zinc atom and two chlorine atoms:
1 zinc atom x 65.38 g/mol = 65.38 g/mol Zn
2 chlorine atoms x 35.45 g/mol = 70.90 g/mol Cl
Total molar mass = 65.38 g/mol + 70.90 g/mol = 136.28 g/mol ZnCl2
Now we can use this molar mass to convert moles of zinc chloride to grams:
1.165 moles ZnCl2 x 136.28 g/mol ZnCl2 = 158.3 grams ZnCl2
Therefore, 2.33 moles of hydrochloric acid reacting completely would produce 158.3 grams of zinc chloride.
Learn more about zinc chloride here:
https://brainly.com/question/26385253
#SPJ1
What type of bonds are shown below?1091AQ1
A. Ionic
B. Polar covalent
C. Nonpolar covalent
D. There is not enough information to determine the answer.
Answers
I'm 99% sure it is A Ionic
Answer: It’s B, Polar covalent
Explanation:
what are the major products (including stereochemistry) of the following reaction? in the reaction scheme, a compound reacts with cl2 in ch2cl2. the reactant contains a ring with six vertices. there is a double bond between the first and the second (clockwise) vertices. a ch3 group is attached to the first vertex. draw the molecule on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars. show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas.
Answers
The major products of the reaction are (1R,2S)-3,4-dichloro-1-methylcyclohexane and (1S,2R)-3,4-dichloro-1-methylcyclohexane, with retention of stereochemistry from the starting material.
The major product of the reaction between the compound with a 6-membered ring, double bond between first and second vertices, and a CH3 group attached to the first vertex, and chlorine (Cl2) in CH2Cl2 is an addition of the two chlorine atoms across the double bond with retention of configuration. This results in a 3,4-dichloro-1-methylcyclohexane product with two possible stereoisomers: (1R,2S)-3,4-dichloro-1-methylcyclohexane and (1S,2R)-3,4-dichloro-1-methylcyclohexane.
The (1R,2S)-stereoisomer is drawn with the wedge bonds on the 1st and 2nd vertices, while the (1S,2R)-stereoisomer is drawn with the dashed bonds on the 1st and 2nd vertices. Both isomers will have the CH3 group on the 1st vertex and the two Cl atoms on the 3rd and 4th vertices.
The product will have the stereochemistry of the starting material, meaning that the molecule with the wedge bonds will have the (1R,2S)-stereoisomer, while the molecule with the dashed bonds will have the (1S,2R)-stereoisomer.
For more such questions on Stereochemistry.
https://brainly.com/question/28658912#
#SPJ11
co3 is ionic or covalent
Answers
which one of the following salts, when dissolved in water, produces the solution with the highest ph? which one of the following salts, when dissolved in water, produces the solution with the highest ph? rbcl rbi rbf rbbr
Answers
Among the salts you mentioned, the one that produces the solution with the highest pH when dissolved in water is RbF (rubidium fluoride).
RbF is a salt formed by the combination of rubidium (Rb) cation and fluoride (F) anion. When it dissolves in water, it undergoes hydrolysis, where water molecules react with the F- anion to produce hydroxide ions (OH-) and HF (hydrofluoric acid). The presence of hydroxide ions increases the pH of the solution, making it more basic or alkaline.
On the other hand, the other salts you mentioned (RbCl, RbI, RbBr) do not undergo significant hydrolysis in water and do not release hydroxide ions to the same extent as RbF. Therefore, their effect on pH is not as pronounced, and the resulting solutions will have a lower pH compared to RbF.
learn more about solution here
https://brainly.com/question/1616939
#SPJ11
Stoichiometry
2. Balance the equation
Name:
1. Given this equation: N₂ +_______ H₂ →NH,, balance it and write the following molar ratios:
a)
N₂/H₂
b)
Ng/NH,
c) Hạ / NH,
a) Li,N/H,O
b)
Answers
N₂ + 3H₂ →2NH\(_3\) is the balanced equation. A balanced equation is indeed a chemical reaction equation.
What is balanced equation?A balanced equation is indeed a chemical reaction equation in which the overall charge and the amount of atoms for every element inside the reaction are the same for both the products and reactants.
In plenty of other terms, the mass as well as charge across both endpoints of the reaction are balanced.
N₂ + H₂ →NH\(_3\)
Number of atom of nitrogen on reactant side is 2 while on product side it is 1.
N₂ + H₂ →2NH\(_3\)
Number of atom of hydrogen on reactant side is 2 while on product side it is 6.
N₂ + 3H₂ →2NH\(_3\)
Therefore, N₂ + 3H₂ →2NH\(_3\) is the balanced equation.
To learn more about balanced equation, here:
https://brainly.com/question/29764227
#SPJ6
Why is it safe to use salt in your food if salt is made of two dangerous elements?
Answers
Because sodium and chlorine on their own have really poisonous/combustive characteristics. However, when they have been chemically bonded together, their characteristics change. They'll be completely harmless. =]
Do NOT ever try a mixture of the two (one that hasn't being chemically combined) it can have fatal results.
25g of potassium nitrate are dissolved in 100g of water. What is the percent by mass of potassium nitrate?
Answers
What is the ratio of the effusion rate of the helium to methane? (round to hundreds)
Answers
Answer:
2.00
Explanation:
From Graham's law; the rate of effusion is inversely proportional to the square root of the relative molecular mass.
Let the rate of effusion of Helium be R1
Let the rate of effusion of Methane be R2
Let the molecular mass of Helium be M1
Let the molecular mass of Methane be M2
Hence;
R1/R2 = √M2/M1
R1/R2 = √16.0294/4.0026
R1/R2 = 2.00
How many grams of H2S is needed to produce 18.00g of PbS if the H2S is reacted with an excess (unlimited) supply of Pb(CH3COO)2?
Answers
Answer:
2.56g of H₂S
Explanation:
The reaction of H₂S with Pb(CH₃COO)₂ is:
H₂S + Pb(CH₃COO)₂ → 2 CH₃COOH + PbS
Where 1 mole of H₂S produce 1 mole of PbS
18.00g of PbS (Molar mass: 239.3g/mol):
18.00g of PbS ₓ (1mol / 239.3g) = 0.0752 moles of PbS
As 1 mole of PbS is produced from 1 mole of H₂S, moles needed of H₂S are 0.0752
As molar mass of H₂S is 34.1g/mol, mass in 0.0752moles is:
0.0752 mol H₂S ₓ (34.1g / mol) = 2.56g of H₂S
a sample of gas at a pressure of 5.1 atm and a temperature of 45 degrees celsius is heated. it’s pressure goes to 7.8 atm what is it’s new temperature in celsius
Answers
Answer:
213.4°C.
Explanation:
Data obtained from the question:
Initial pressure (P1) = 5.1atm
Initial temperature (T1) = 45°C = 45°C + 273 = 318K
Final pressure (P2) = 7.8atm
Final temperature (T2) =..?
The new temperature can be obtained as follow:
P1/T1 = P2 /T2
5.1/318 = 7.8/T2
Cross multiply
5.1 x T2 = 318 x 7. 8
Divide both side by 5.1
T2 = 318 x 7. 8 /5.1
T2 = 486.4K
Finally, we shall convert 486.4K to celsius temperature. This can be done as follow:
T(°C) = T(K) – 273
T(K) = 486.4K
T(°C) = 486.4 – 273
T(°C) = 213.4°C.
Therefore, the new temperature is 213.4°C
An unknown substance is placed in a graduated cylinder of water. The substance immediately sinks to the bottom. What could the density of the substance be?
Answers
If a liquid is less dense than the liquid it is placed in, it will float. An unidentified material is added to a graded water cylinder. The stuff hits the bottom right away. Mass/volume equals density.
When placed in water, an object will float if its density is lower than that of the water, whereas it will sink if its density is higher. The density of a material is a distinguishing quality that is independent of the substance's volume. This assertion is supported by the Archimedes principle. This is due to the fact that the buoyant force pulling on the object is smaller than its weight. The object floats on the liquid's surface if its density is less than or equal to that of the liquid.
Read more about unknown substance at
https://brainly.com/question/2384930
#SPJ4
HOW IS PHENOTYPE DIFFERENT THAN GENOTYPE?
Answers
Answer: The genotype is the set of genes in our DNA which is responsible for a particular trait. The phenotype is the physical expression, or characteristics, of that trait. For example, two organisms that have even the minutest difference in their genes are said to have different genotypes.
While we wait for the incubation, let's think about what we expect. If 1 ml of the initial solution contained one million cells, how many colis would you expect in 1 ml of the 0.0001 dilution? a) 100 b) 1 C) 10.000 d) 10
Answers
Based on the dilution, we can expect there to be 10 colis in 1 ml of the 0.0001 dilution of the initial solution.
The initial solution contained one million cells in 1 ml. The 0.0001 dilution is 10,000 times smaller than the initial solution. Therefore, we can calculate the number of cells in the 0.0001 dilution by dividing one million by 10,000, which gives us 100 cells. However, the question specifically asks for colis, which are a type of bacteria commonly used in microbiology. We can assume that all one million cells in the initial solution were colis, so the proportion of colis in the 0.0001 dilution should be the same. Therefore, we can expect there to be 10 colis in 1 ml of the 0.0001 dilution.
To fully understand the calculation, we need to consider the concept of dilutions in microbiology. Dilutions are used to reduce the concentration of bacteria in a sample, making it easier to count and analyze them. In this case, we are given an initial solution containing one million cells in 1 ml. This solution is then diluted by a factor of 10,000, resulting in a 0.0001 dilution. This means that for every 1 ml of the 0.0001 dilution, there is only 1/10,000th of a ml of the original solution.
To calculate the number of cells in the 0.0001 dilution, we need to determine the proportion of cells that were carried over in the dilution. Since the dilution factor is 10,000, we can divide the number of cells in the initial solution by 10,000 to get the number of cells in the 0.0001 dilution. This gives us 100 cells in 1 ml of the 0.0001 dilution. However, the question asks for the number of colis, which are a specific type of bacteria. Since we know that the initial solution contained one million colis, we can assume that all of the cells in the 0.0001 dilution are colis as well. Therefore, we can expect there to be 10 colis in 1 ml of the 0.0001 dilution.
In summary, based on the dilution factor of 10,000, we can expect there to be 100 cells in 1 ml of the 0.0001 dilution. Since all of the cells in the initial solution were colis, we can assume that all of the cells in the 0.0001 dilution are colis as well. Therefore, we can expect there to be 10 colis in 1 ml of the 0.0001 dilution.
To know more about dilution, visit:
https://brainly.com/question/28548168
#SPJ11
Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help Help

Answers
The reaction is run at two temperatures where temperature 1 is lower than temperature 2. Which relationship is correct for either rate constant (k1 or k2) or activation energy (Ea,1 and Ea,2)
Answers
Answer:
The answer is "\(K_1 < K_2\)"
Explanation:
In the given question, the value of the \(K=Ae^{-\frac{Ea}{RT}}\) and the \(T_1 <T_2\)is the rate value which is the constant that is \(K_2>K_1\). As per the temperature value when its increase rate is constantly increasing. \(E_a\) is activation energy it is not dependent on the temperature that why the answer is \(K_1 < K_2\).
Which of the following is the correct reaction for enthalpy of formation of ozone at 25 °C?
A. O2(g) + O(g) → O3(g)
B. 3/2O2(g) → O3(g)
C. O(g) + O(g) +O(g) → O3(g)
D. 3O2(g) → 2O3(g)
E. O3(g) → O3(g
Answers
The correct reaction for the enthalpy of formation of ozone at 25 °C is D. 3O2(g) → 2O3(g).
The reaction D represents the formation of ozone (O3) from molecular oxygen (O2) gas. This reaction involves three molecules of O2 combining to form two molecules of O3. The enthalpy of formation is defined as the change in enthalpy when one mole of a substance is formed from its constituent elements in their standard states. In this case, the standard state of oxygen is O2(g), and the standard state of ozone is O3(g).
The reaction D correctly shows the stoichiometry of the formation of ozone, where three molecules of O2 react to produce two molecules of O3. This balanced equation represents the enthalpy change associated with the formation of ozone and is consistent with experimental observations and thermodynamic data.
Learn more about enthalpy here: brainly.com/question/29145818
#SPJ11
the martian polar caps are thought to be mainly frozen methane and ammonia.
true or false
Answers
False. The statement that the Martian polar caps are mainly frozen methane and ammonia is false. The Martian polar caps primarily consist of water ice and carbon dioxide (CO₂) ice.
The Martian polar caps, also known as the Martian ice caps, are composed mainly of water ice. These caps are analogous to Earth's polar ice caps and contain a significant amount of frozen water. The water ice at the Martian poles is mixed with carbon dioxide ice, which forms a seasonal cap of dry ice during the colder Martian winters.
Methane (CH₄) and ammonia (NH₃) are present in trace amounts in the Martian atmosphere, but they are not the primary constituents of the polar caps.
Methane on Mars is a particularly interesting topic of study, as its presence has been detected and is attributed to various potential sources such as geological activity or microbial life. However, it is not a dominant component of the polar caps.
So, the main constituents of the Martian polar caps are water ice and carbon dioxide ice, while methane and ammonia are present in smaller quantities in the Martian atmosphere but do not primarily compose the polar caps.
Learn more about Martian polar caps here:
https://brainly.com/question/32264467
#SPJ11
Select the correct answer from the drop-down menu.

Answers
If it takes 1.33 seconds for radio waves (which travel at the speed of light) to reach the moon from Earth, how far away is the moon?
Answers
Speed of light * time = distance
299,792,458 * 1.33 = 398,723,969 meters
398,723.969 km to the moon
If the radio waves from earth reach moon within 1.33 s in a speed of light then, the distance of moon from earth will be 3.9 ×10⁸ m.
What are radio waves?Radio waves are electromagnetic waves with highest wavelength and lesser energy. The speed of these waves will be equal to the light speed that is, 3 ×10⁸ m/s.
Distance is the product of time and velocity. It is given that the time taken by the radio wave to reach moon from earth is 1.33 seconds and the velocity of wave is 3 ×10⁸ m/s thus, distance to moon is calculated as follows:
Distance = Time × velocity
= 1.33 s × 3 ×10⁸ m/s
= 3.9 ×10⁸ m
Hence, moon is 3.9 ×10⁸ m away from earth.
To learn more about radio waves?
https://brainly.com/question/13989450
#SPJ2
Pls help will give brainlist
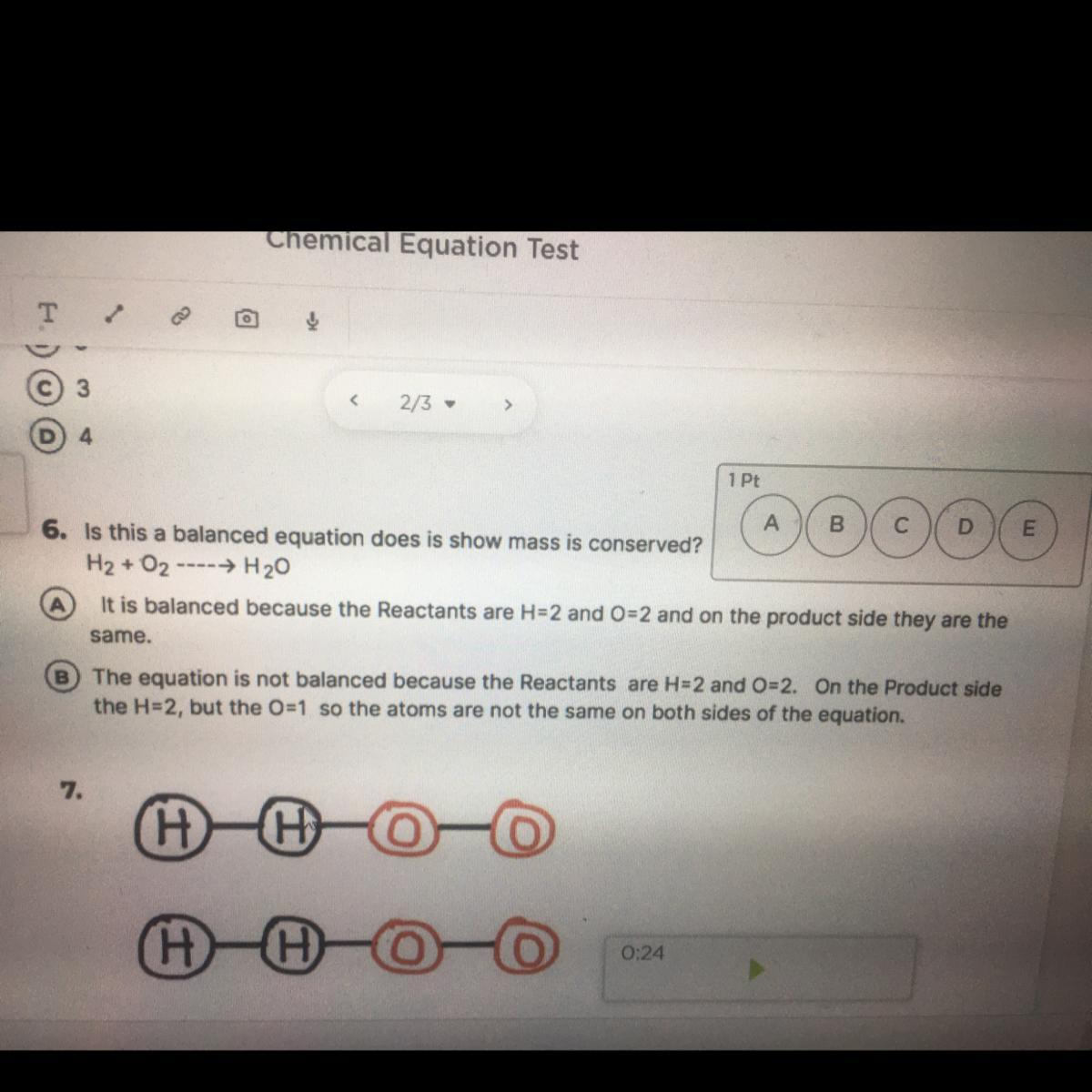
Answers
Answer:
B
Explanation:
H2O is 2 hydrogen atoms, 1 oxygen atom. H2+O2 would yield H2O2, not H2O. The balanced equation is 2H2+2O2=2H2O
Each of these Ionic Compounds are named INCORRECTLY. 1. Find and describe the mistake 2. Correctly name the compound. CaCl2 - Calcium Chlorine CuS - Copper Sulfide Li3P - Trilithium Phosphide CuBr - Copper(II) Bromide NaOH - Sodium Hydrogen Oxide
Answers
Explanation:
The nomenclature of ionic compounds is given by:
1. Positive is written first. And if the metal atom has various oxidation states then its oxidation state is to be mentioned in brackets with help of roman numbers
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
1.) : Calcium chloride (Correct)
In the given compound, calcium has oxidation state of +2 and chlorine has oxidation state of -1.The name is correct.
2.) : Copper (II) oxide (Incorrect)
In the given compound,copper has the oxidation state of +1 and oxygen has oxidation state of -2.So ,the correct name will be Copper (I) oxide.
3.) : Lead (II) sulfide (Incorrect)
In the given compound, lead has the oxidation state of +4 and sulfur has oxidation state of -2. So ,the correct name will be Lead (IV) sulfide.
A magnet was placed near a pile that contained both iron and sulfur. The magnet was moved gradually closer to the pile. As it neared the pile, the magnet started attracting small pieces of iron from the pile. Which of these statements best describes the contents of the pile?
Answers
The question is incomplete, the complete question is;
A magnet was placed near a pile that contained both iron and sulfur. The magnet was moved gradually closer to the pile. As it neared the pile, the magnet started attracting small pieces of iron from the pile. Which of these statements best describes the contents of the pile?
F. It is a homogeneous mixture of iron and sulfur. G. It is a heterogeneous mixture of iron and sulfur. H. It is a compound that contains both iron and sulfur. I. It is a compound that can be separated by magnetism.
Answer:
G. It is a heterogeneous mixture of iron and sulfur.
Explanation:
A heterogeneous mixture is one that does not have a uniform composition throughout.
We must recall that a mixture is any combination of substances that do not chemically react together and are separable by physical means.
Having said this, it is clear that I can separate the iron from sulphur by simple magnetic (physical) means. Hence, it is a heterogeneous mixture of iron and sulfur.
Answer:
It is a heterogeneous mixture of iron and sulfur.
Explanation: