Answers
The rule that helps you predict the formulas of ionic compounds formed between metal and nonmetal elements is by determining the charges of the ions by using the periodic table. Metal elements tend to lose electrons and form positive ions, while nonmetal elements tend to gain electrons and form negative ions.
Determine the ratio of cations to anions required to produce a neutral compound. This can be accomplished by finding the lowest common multiple of the charges of the ions and utilizing that as the ratio. Write the formula taking up symbols of the elements and the suitable ratio of ions. For example, if the cation is magnesium and the anion is chloride, the formula for the compound would be MgCl₂, implying that there are two chloride ions for every magnesium ion.
But this is simply a general rule and there may be exceptions.
Learn more about ionic compounds here:
https://brainly.com/question/1450764
#SPJ4
Related Questions
Calculate the lattice energy of CuBr(s) using a Born–Haber cycle.
Data:
Enthalpy of sublimation of Cu(s) = 337.7 kJ/mol
1st ionization energy of Cu(g) = 745 kJ/mol
Enthalpy of vaporization of Br2(l) = 29.96 kJ/mol
Answers
Answer- 2nd
Calculate the partial
pressure of argon in a
gas mixture with a total
pressure of 2.4 atm.
The partial pressures
of the other gases are:
O2 = 128.0 mmHg
He = 167.5 mmHg and
Ne= 760.0 mmHg
Answers
The required partial pressure of argon gas present in the mixture is 1.02 atm.
What is Dalton law of gas?Dalton's law of gas states that total pressure of any mixture of gas is sum of the partial pressure of all the gases present in that mixture.
Given that,
Total pressure of mixture = 2.4 atm
Partial pressure of argon = ?
Partial pressure of oxygen = 128 mmHg = 0.168 atm
Partial pressure of helium = 167.5 mmHg = 0.220 atm
Partial pressure of neon = 760 mmHg = 1 atm
On putting all these values according to the definition we get the partial pressure of argon gas as:
Partial pressure of argon = 2.4 - (0.168 + 0.22 + 1) = 1.02 atm
Hence required partial pressure of argon gas is 1.02 atm
To know more about Dalton's law, visit the below link:
https://brainly.com/question/8040766
#SPJ1
Identify the false statement from the following.
A. London dispersion forces exist in all polar molecules.
B. Temporary charge imbalances in the molecules lead to London dispersion forces.
C. Hydrogen bond is type of dipole – dipole interaction.
D. London dispersion is the weakest among the intermolecular forces.
Answers
The false statement from the above is that: Temporary charge imbalances in the molecules lead to London dispersion forces.
What are the factors that affect London dispersion forces?Generally, the factors which affects the London dispersion forces a dispersion force are as follows:
Shape of the moleculesDistance between moleculesPolarizability of the moleculesHowever, London dispersion forces simply refers to a sort of temporary attractive force formed when electrons in two adjacent atoms occupy positions that make the atoms form dipoles.
So therefore, temporary charge imbalances in the molecules lead to London dispersion forces is a false statement
Learn more about London dispersion forces:
https://brainly.com/question/1454795
What is paper made of?
Answers
Paper used as a writing material is made of pulp (wood).
What is paper?Paper is a sheet material used for writing on or printing on (or as a non-waterproof container), usually made by draining cellulose fibres from a suspension in water.
Paper is made from cellulose found in trees, which are the main source of cellulose fibre (or woodpulp). Besides woodpulp, paper can be made from other materials such as cotton, flax, esparto, straw, hemp, manilla and jute.
Wood pulp is usually a softwood, used for pulping to make paper.
Learn more about pulp at: https://brainly.com/question/23590026
#SPJ1
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
Briefly identify a few characteristic points about the following bonds:
a. Ionic bonds
b. Covalent bonds
c. Hydrogen bonds.
Answers
Answer:
Chemical bonds hold molecules together and create temporary connections that are essential to life. Types of chemical bonds including covalent, ionic,
Explanation:
Ionic bond will form by transferring the electrons , covalent bond formed by sharing the electrons while hydrogen bond will be formed by bond between electronegative atom and hydrogen molecule.
What is ionic bond?
The chemical bond is formed when electrons are transferred from one atom to another. An ionic bond is the name for this type of relationship. Their melting and boiling points are quite high. Ionic chemicals, in other words, seem to be non-volatile.
What is covalent bond?When one non-metal reacts with just another non-metal, electrons are shared across their atoms, resulting in the development of a covalent bond. Among two or more atoms with same non-metal, a covalent connection can be established.
What is hydrogen bond?Hydrogen bonding is just a sort of dipole-dipole attraction that occurs between molecules rather than a covalently link with a hydrogen atom.
To know more about hydrogen bond , covalent bond and ionic bond,
https://brainly.com/question/11527546
#SPJ2
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
In Thomas Cole's The oxbow who is the one figure depicted in the landscape and what is he doing
Answers
Answer:
In Thomas Cole's painting "The Oxbow," Thomas Cole, the artist, shows himself sitting in the landscape while painting it.
Explanation:
In the painting called "The Oxbow" by Thomas Cole, the artist actually put himself in the picture. He's sitting right in the middle of the landscape, just like he placed himself in the painting, as if taking a selfie while working on it. It's like he took a snapshot of himself while he was working on the painting. This shows how much he cared about creating the artwork and how he felt a personal connection to the beautiful scenery. It's really fascinating because it gives us a glimpse into how he saw himself as an artist and how he wanted to capture the incredible beauty of nature through his art.
In the compound Fe2O3, iron's oxidation number is +3, and oxygen's oxidation
number is
Answer here
Answers
Answer: The oxygen's oxidation number is -2.
Explanation:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
In \(Fe_2O_3\), Fe is having an oxidation state of +3 called as cation and oxygen is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral \(Fe_2O_3\)
The cations and anions being oppositely charged attract each other through strong coloumbic forces and form an ionic bond.
Which type of energy would running be?
Thermal
Light (solar)
Kinetic
Potential
Answers
Answer:
so here is the answer
Explanation:
Kinetic is the answer.
I really need to know the answer it will help

Answers
Answer:
so the answer is A 5.199mol please. rate. me brainliest. if its. correct
Explanation:
N=nL
\(3.131 \times {10}^{24} = n \times 6.022 \times {10}^{23} \\n = \frac{3.131 \times {10}^{24} }{6.022 \times {10}^{23} } \\ n = 5.199mol\)
You have two containers. The first container contains 22.99 grams of solid
sodium (Na). The second container contains 15.999 grams of Oxygen (O).
Which container has more moles of the indicated substance? *
Answers
Answer:
The sodium has more moles.
n(Na)=2.09 mole
n(O)=0.999 mole

How many L of 4.0 M solution can be made with 132g of NaCI ?
A)9.02L
B)9.02ml
C)0.9L
Answers
Answer:
0.430 Litre
Explanation:
The first thing that you need to do here is to convert the mass of lithium bromide to moles by using the compound's molar mass.\(100g \frac{1 moleNaCl}{58.5g} = 1.71 moles of NaCl\)
Now, the molarity of the solution is simply a measure of the number of moles of solute, which in your case is NaCl, present for every 1.00 L of the solution.
In order to have a 4.00-M solution of NaCl, you need to have 4.00 moles of NaCl for every 1.00 L of this solution.
You know that your sample contains 1.71 moles of NaCl, so you can use the molarity of the solution as a conversion factor to determine how many liters of this solution can be made.
\(1.71 mole NaCl \frac{ 1 L Solution}{4 mole NaCl} = 0.43 L solution\).
Trend of atomic number and atomic size of the elements when we move from left to right in different periods of periodic table
Answers
Answer:
The atomic size decreases with an increase in atomic number when we move from left to right.
Explanation: Hope it helps you:))))))
Have a great day.
Determine if each statement is an acid, base, or neutral:- donates an OH- ion- donates an H+ ion- removes OH- ions from water- removes H+ ions from water
Answers
Answer:
\(\begin{gathered} a)\text{ Base} \\ b)\text{ Acid} \\ c)\text{ Acid} \\ d)\text{ Base} \end{gathered}\)Explanation:
Here, we want to determine if each statement is an acid, a base or neutral
a) Donates an OH- ion
In the reaction of an acid with a base, the acid ionizes and donates the hydrogen ion while the base donates the hydroxide ion
What that means is that, it is the base that donates the hydroxide
b) Donates an H+ ion
In the reaction of an acid and a base, it is the acid that donates, the hydrogen ion. The answer here is the acid
c) removes OH- ions from water
An acid releases H+ ions in water and thus takes OH- ions. Thus, it is an acid that removes OH- ions from water
d) Removes H+ ions from water
A base releases OH- ions and thus abstracts H+ ions from water
Why was the morning session stopped unsuccessfully?
The reactor was overheating.
The automatic control system was not adjusted properly.
The vernier control rod became stuck.
The emergency rod failed.
Answers
Answer:
the reactor was overheating
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).How many grams of potassium iodide (KI) are required to prepare 50 mL of a 0.30 M
solution?
Answers
Answer:
137 g
Explanation:
Since1 L=103 mLyou can say that your sample must contain 550.0mL solution⋅1.50 moles KI103mL solution=0.825 moles KITo convert the number of moles of potassium iodide to grams, use the molar mass of the compound.0.825moles KI⋅166.003 g1mole KI=137 g−−−−−
__Hgo > __Hg + __O2?
Answers
Hg + O2 → HgO
✅Word equation: Mercury + Oxygen gas → Mercury (II) oxide
✅ Type of Chemical Reaction: For this reaction we have a combination reaction.
✅ Balancing Strategies: To balance this equation it's probably easiest to begin by changing the coefficient in front of the HgO.
This is a combination reactions because the mercury (Hg) plus the oxygen gas (O2) come together to form the Mercury (II) oxide (MgO).
Hint-1
Hint-2
IamSugarBee
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol.
Answers
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol, the molecular formula is NO₂.
We must compute the empirical formula in order to ascertain the compound's chemical composition.
If we have 100 grams of the compound.
This suggests we have:
30.4 g of nitrogen
69.6 g of oxygen
Now, we have to convert the mass of each element to moles.
The molar mass of nitrogen (N) = 14.01 g/mol
the molar mass of oxygen (O) = 16.00 g/mol.
Number of moles of nitrogen (N):
2.17 mol
Number of moles of oxygen (O):
4.35 mol
The simplest whole-number ratio between the moles of nitrogen and oxygen must now be determined. To calculate the ratio, we divide both numbers by the smaller value.
Moles N / moles O = 2.17 mol / 2.17 mol = 1.00
Moles O / moles O = 4.35 mol / 2.17 mol = 2.00
The ratio is approximately N₁O₂.
We divide the subscripts by their greatest common divisor to obtain the simplest ratio, since we are looking for the empirical formula. The empirical formula is NO₂ since the greatest common divisor in this situation is 1.
The molecular formula of the compound is NO₂.
Learn more about molecular formula, here:
https://brainly.com/question/11203434
#SPJ1
What is the main function of the part labeled Y in the model?
A To hold and protect the cell's DNA
B. To store enzymes that break down food
C. To store food and other materials inside a cell
D. To use energy from sunlight to make sugars
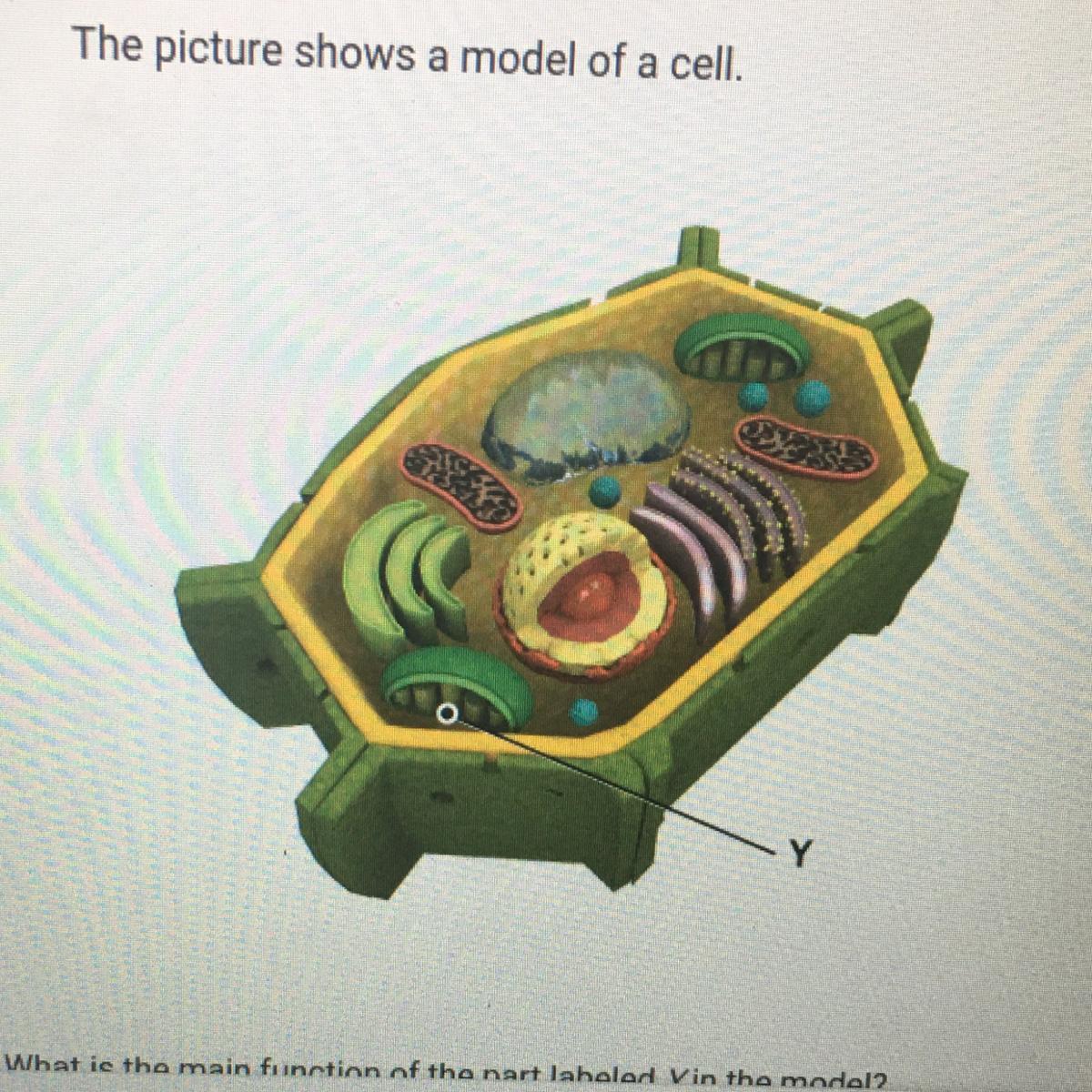
Answers
Answer
D
Explanation
the answer is D
Answer:
The answer is D.To use energy from sunlight to make sugars
Explanation:
Hope this helps <3
Cells are made of cells true or false
Answers
false
cells are made up of nucleus and cytoplasm and it's contained within the cell membrane
What are 3 different ways we can preserve our ecosystem and what makes your 3 ways effective?
Answers
Answer:
1. Stop throwing trash and bottles in lakes because it can have our animals live longer and not have issues
2. Reducing the number of bags you use from the store because they can end up being on the ground and cause plenty of damage around us
3. probably trying not to having fires because there is alot of problems that are happening like the wildfires in californa which is leading to our animals and trees dying
hope this helps
have a good day :)
Explanation:
Complete the table:
Mass Solute Mass Solvent Mass Solution Mass Percent
2.65 g 26.0 g _____ _____
_____ 46.8 g _____ 4.0 %
1.68 g _____ 26.2 g _____
26.7 g _____ _____ 5.9 %
Answers
Answer:
1.
Mass solution = 28.65 g
Mass percent = 9.14 %
2.
Mass solute = 1.95 g
Mass solution = 48.75 g
3.
Mass solvent = 24.52 g
Mass percent = 6.41 %
4.
Mass solution = 476.8 g
Mass solvent =450.1 g
Explanation:
Hello,
In this case, we define the mass percent as:
\(\%m/m=\frac{m_{solute}}{m_{solute}+m_{solution}} *100\%\)
Wherein we use the mass of the solute and the mass of the solvent in order to compute it, thus, for each case we have:
1.
Mass solution: 2.65 g + 26.0 g = 28.65 g
Mass percent: 2.62 g / (2.65 g + 26.0 g) x 100% = 9.14 %
2.
Mass solute: 4.0 % x 46.8 g / (1 - 4.0 %) = 1.95 g
Mass solution: 1.95 g + 46.8 g = 48.75 g
3.
Mass solvent: 26.2 g - 1.68 g = 24.52 g
Mass percent: 1.68 g / (1.68 g + 24.52 g) x 100% = 6.41 %
4.
Mass solution: 26.7 g / 5.6 % = 476.8 g
Mass solvent: 476.8 g - 26.7 g =450.1 g
Best regards.
Answer:
20.9
Explanation:
The standard enthalpies of combustion of fumaric acid and maleic acid (to form carbon dioxide and water) are - 1336.0 kJ moJ-1 and - 1359.2 kJ moJ-1, respectively. Calculate the enthalpy of the following isomerization process:
maleic acid ----> fumaric acid
Answers
Answer:
Explanation:
maleic acid ⇒ fumaric acid
ΔHreaction = ΔHproduct - ΔHreactant
ΔHproduct = -1336.0 kJ mol⁻¹
ΔHreactant = - 1359.2 kJ mol⁻¹.
ΔHreaction = -1336.0 kJ mol⁻¹ - ( - 1359.2 kJ mol⁻¹.)
= 1359.2 kJ mol⁻¹ -1336.0 kJ mol⁻¹
= 23.2 kJ mol⁻¹ .
Enthalpy of isomerization from maleic to fumaric acid is 23.2 kJ per mol.
16) Select the best answer.
Round the answer to the correct number of significant figures.
10.05
2.8899 = 29.043495
29.0435
29.04
29.043
29
Answers
29 is not the best answer depends on the context and the rules for significant figures.
What is best answer?
The best answer depends on the context and the rules for significant figures. If we assume that we need to round to three significant figures:
10.05 has three significant figures, so it is already rounded correctly.2.8899 has four significant figures, so we need to round it to three significant figures. The third significant figure is 9, which is greater than 5, so we round up the second significant figure (which is 8) to 9. Therefore, 2.8899 rounded to three significant figures is 2.89.29.0435 has five significant figures, so we need to round it to three significant figures. The third significant figure is 0, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.0435 rounded to three significant figures is 29.0.29.04 has four significant figures, so it is already rounded correctly.29.043 has four significant figures, so we need to round it to three significant figures. The third significant figure is 3, which is less than 5, so we do not round up the second significant figure (which is 4). Therefore, 29.043 rounded to three significant figures is 29.0.29 has one significant figure, so it is not rounded correctly to three significant figures.Therefore, 29 is not the best answer.
To know more about significant figures, visit:
https://brainly.com/question/30465808
#SPJ1
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Given a titration reaction, the titrant concentration and volume, and the analyte volume and mass, identify the titrant to analyte stoichiometry
Answers
Given a titration reaction, titrant concentration and volume, the analyte volume and mass, then to identify titrant to analyte stoichiometry : Put the coefficients of each compound in order and simplify if possible.
How do you identify analyte and titrant?During titration, there are two solutions: analyte and the titrant. Analyte is the unknown solution for which you would like to know the concentration or the equilibrium constant. The titrant is the known solution which has a precise and accurate concentration.
The stoichiometric point is the point where an equal amount of acid and base is present to neutralize chemical reaction. Known reagents added to chemical reaction are called titrant and unknown solution is called analyte.
To know more about titration, refer
https://brainly.com/question/186765
#SPJ4