Answers
Related Questions
Samples of two metals of equal mass but with different heat capacities are originally at the same temperature. Il the same amounto1'heat is added to both samples, for which metal will the final temperature be lower (assume that no phase change, such as meltng, occurs).
Answers
The heat capacity corresponds to the energy needed to raise one degree of temperature for one gram of substance. That is, the greater the heat capacity, for the same mass, the greater the energy required to raise the temperature of the material.
Therefore, between the two metals with the same mass, the same initial temperature, and the same heat added, we can say that the one with the higher heat capacity will present a lower final temperature.
Please help!!
Is this an:
Acid
Base
Neither
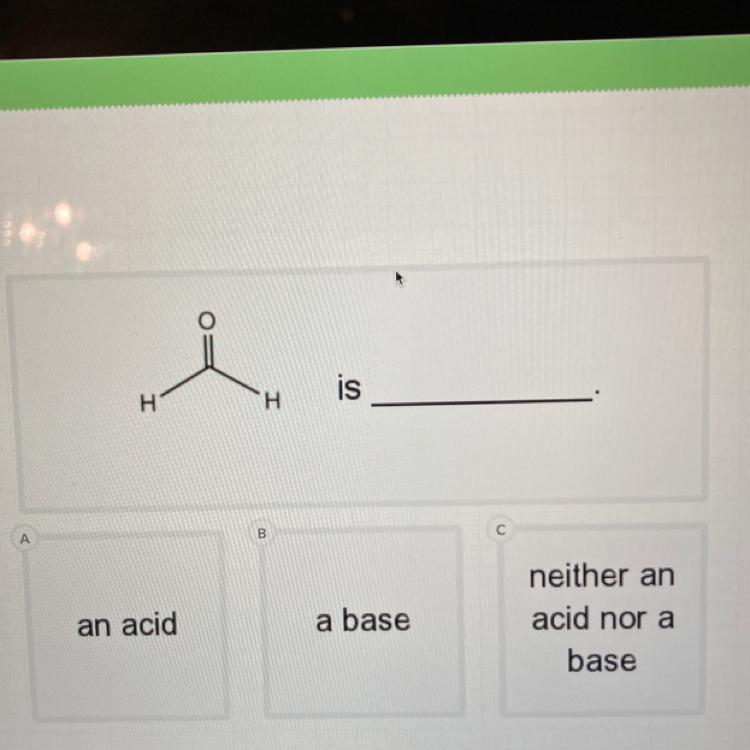
Answers
Answer:
it is a weak acid
acid
Explanation:
formaldehyde is a weak acid (pK greater than 13),
Opinión personal sobre El Planeta
Answers
la tierra nuestro planeta es el único del sistema solar que sabe que mantiene vida: vida que es increíblemente diversa,
What’s the answer for this question which i have posted can I know the answers as possible

Answers
The change in internal energy = 2.701 x 10² kJ
Further explanationGiven
Heat absorbed by system = 1.69 x 10² kJ
Work done on system = 1.011 x 10² kJ
Required
The change in Internal energy
Solution
We can use the first law of Thermodynamics :
\(\tt \Delta U=Q+W\)
The sign rules for heat and work are set as follows:
• The system receives heat, Q +
• The system releases heat, Q -
• The system does work, W -
• the system accepts work, W +
Heat absorbed/receive heat from surrounding = Q+
Work done on the system = W+
and input the given values
\(\tt \Delta U= 1.69\times 10^2+1.011\times 10^2\\\\\Delta U=\boxed{\bold{2.701\times 10^2~kJ}}\)
How is this compound classified C4H6O4
Answers
The compound C4H6O4 can be classified as a dicarboxylic acid. In this case, the presence of four carbon atoms (C4) indicates that it is a relatively larger molecule.
The molecular formula also contains six hydrogen atoms (H6) and four oxygen atoms (O4). The presence of oxygen and carbon atoms suggests the possibility of carboxyl groups (-COOH) in the compound. Carboxyl groups are functional groups consisting of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom.
Since the compound contains four oxygen atoms, it is possible that it contains two carboxyl groups. A compound with two carboxyl groups is classified as a dicarboxylic acid. Dicarboxylic acids are organic compounds that have two carboxyl functional groups.
They are characterized by their ability to donate two protons (H+) and act as acids. Therefore, based on the molecular formula C4H6O4, the compound is classified as a dicarboxylic acid.
For more such questions on compound
https://brainly.com/question/15929599
#SPJ11
A gas cylinder initially contains 463 L of gas at a pressure of 159 atm. If the final volume of gas is 817 L, what is the final pressure?
Answers
Answer:
The final pressure is 90.1 atm.
Explanation:
Assuming constant temperature, we can solve this problem by using Boyle's Law, which states:
P₁V₁=P₂V₂Where in this case:
P₁ = 159 atmV₁ = 463 LP₂ = ?V₂ = 817 LWe input the given data:
159 atm * 463 L = P₂ * 817 LAnd solve for P₂:
P₂ = 90.1 atmThe final pressure is 90.1 atm.
What is the pressure in atmospheres exerted by a column of water that is 12.5 m
high?
(density of water = 1.00g/cm3; gravitational constant = 9.80665 m/s2, 1 atm =
101,325 Pa = 760 torr = 760 mmHg)
Answers
Answer:
0.00121 atm
Explanation:
Step 1: Given data
Height of the column of water (h): 12.5 mDensity of water (ρ): 1.00g/cm³Gravitational constant (g): 9.80665 m/s²Step 2: Calculate the pressure exerted by the column of water in SI units
The pressure exerted by the column of liquid depends on the height of the column, the density of the liquid and the gravitational constant. We can calculate it using the following expression.
P = ρ × g × h
P = 1.00g/cm³ × 9.80665 m/s² × 12.5 m
P = 123 Pa
Step 3: Convert "P" to atm
We will use the conversion factor 101325 Pa = 1 atm.
123 Pa × (1 atm/101325 Pa) = 0.00121 atm
A student made a sketch of a potential energy diagram to represent a reaction with a -^H
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain
what the values of X and Y represent.

Answers
Answer: It’s correct because it’s showing an exothermic reaction. x is the reactants and y is the products.
Explanation: -ΔH means the reaction is exothermic and releasing heat. This lowers the potential energy.
Answer:
answer above is correct
Explanation:
Which of the following most likely happens when the number of particles of a gas decreases?
Answers
What is the empirical formula for a compound that has 57.5%
Na, 40.0% 0, and 2.5% H?
a
Naz(OH)4
b
Na(OH)2
С
Na(OH)
d
Naz(OH)
Answers
Answer:
c
Explanation:
use %composition given to calculate emperical 4mular
The empirical formula of the compound having 57.5% Na, 40.0% O, and 2.5% H is NaOH (Option C)
Data obtained from the questionNa = 57.5%O = 40%H = 2.5%Empirical formula =?How to determine the empirical formulaDivide by their molar mass
Na = 57.5 / 23 = 2.5
O = 40 / 16 = 2.5
H = 2.5 / 1 = 2.6
Divide by the smallest
Na = 2.5 / 2.5 = 1
O = 2.5 / 2.5 = 1
H = 2.5 / 2.5 = 1
Thus, the empirical formula of the compound is NaOH
Learn more about empirical formula:
https://brainly.com/question/24297883
#SPJ2
Which image best represents the particles in liquids

Answers
Answer:
The second picture
Explanation:
The first picture shows a solid because the particles of a solid are organized and compact. They can only vibrate in place
The third picture represents a gas because of how spaced out each particle is. They can move fast and erratically.
The second picture is the liquid because the particles aren't extremely packed, so they have space to move.
Answer:
The second one is correct
How does shaking or stirring a mixture of solute and solvent affect a solution?
CA. It increases the solubility of the solute.
CB. It increases the rate of dissolving.
C C. It decreases the solubility of the solute.
D. it decreases the rate of dissolving.
Answers
What temperature units
should be used when
performing calculations using
Charles's Law?

Answers
Answer:
Kelvin (K) onlyExplanation:
Kelvin is preferred for solving problems related to Charles' Law because , it is the Absolute temperature scale.
To convert Celsius to Kelvin scale, you add 273 to the temperature in the centigrade/Celsius scale.
What pressure will be produced when 2.0 moles of N2 gas is heated to 68oC in a container that holds 1.25 of gas?
Answers
The pressure of the nitrogen gas produced is determined as 44.77 atm.
What is the pressure of the Nitrogen gas?
The pressure of the nitrogen gas is determined from ideal gas equation, as shown below;
PV = nRT
P = nRT/V
where;
n is number of moles = 2 molesR is ideal gas constant = 0.08205 L.atm/mol.KT is temperature = 68⁰C = 68 + 273 = 341 KV is volume = 1.25 LP = (2 x 0.08205 x 341)/(1.25)
P = 44.77 atm.
Learn more about pressure here: https://brainly.com/question/25736513
#SPJ1
In the barium chloride laboratory activity, what change occurred in the physical appearance of the barium chloride during the heating process?
A. Barium chloride changed from sparkly white to dull white.
B. Barium chloride changed from dull white to sparkly white.
C. Barium chloride changed from sparkly yellow to dull yellow.
D. Barium chloride changed from dull yellow to sparkly yellow.
Answers
Barium chloride turned from sparkly white into dull white during the heating process.
Barium chloride: What is it?An inorganic substance with the formula BaCl2 is barium chloride. It is among the most popular barium salts that dissolve in water. Like the majority of some of the other water-soluble barium salts, is also white, extremely hazardous, and gives flames a yellow-green tint.
What results from consuming barium chloride?Among the most common barium salts is barium chloride. Bacl2 is hygroscopic and soluble in water. Deep hypokalemia, generalized muscle weakness, and eventually paralysis of the limbs and breathing muscles can occur within 1 to 4 hours of consumption.
To know more about barium chloride visit :
https://brainly.com/question/2572464
#SPJ1
Answer the questions based on the following structural diagram: OCH2CH3 CH3CCH2CH3 OCH2CH3 1. The substance is a(n) 2. The substance was formed from a(n) 3. The IUPAC name of the parent aldehvde or ketone is
Answers
Answer:
1. The substance is an ether.
2. The substance was formed from an alcohol and an alkyl halide.
3. The IUPAC name of the parent aldehyde or ketone is propanone.
Explanation:
The given structural diagram represents an ether with the formula CH3C(OCH2CH3)2.
This substance is an ether because it contains an oxygen atom bonded to two alkyl groups. It can be formed from an alcohol and an alkyl halide through a reaction called the Williamson ether synthesis.
The alcohol would be ethanol (CH3CH2OH) and the alkyl halide would be 1-chloropropane (CH3CH2CH2Cl).
The reaction would be as follows:CH3CH2OH + CH3CH2CH2Cl → CH3C(OCH2CH3)2 + HCl
The parent aldehyde or ketone in this case is propanone (CH3COCH3), also known as acetone. This is because the ether is derived from the substitution of the two hydrogen atoms on the carbonyl group of propanone with two ethoxy groups (OCH2CH3). The IUPAC name of the ether would be 2,2-diethoxypropane.
To know more about ether, refer here:
https://brainly.com/question/28047849#
#SPJ11
write the atomicity of oxygen
Answers
Which of these pairings with create an octet for each atom?
A. One aluminum atom and one oxygen atom
B. One magnesium and one chlorine
C. One magnesium and one oxygen
D. One potassium and one sulfur atom
Answers
Answer:
C) one magnesium and one oxygen
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
10. What do you have to do to the coefficients of equation I below to get to equation II?
i. 2 SnO2 + 4 H2 2 Sn + 4 H2O
ii. SnO2 + 2 H2 Sn + 2 H2O
Answers
2/2 = (1) SnO2
4/2 = 2H2
2/2 = (1) Sn
4/2 = 2 H20
Fun fact: the coefficient is called the stoichometric coefficient!!
If antique brass is 30.0% zinc and 70.0% copper, how many grams of antique brass can be made from 18.0 grams of zinc and 32.8 grams of copper?
Please show work! It’s for chem
Answers
Answer: 51.86
Explanation:
Total, 46.86 grams of antique brass can be made from 18.0 grams of zinc and 32.8 grams of copper.
To find out how much antique brass can be made from the given amounts of zinc and copper, we need to determine the limiting reactant in the reaction between zinc and copper.
Calculate the number of moles for each element.
Given:
Mass of zinc (Zn) = 18.0 grams
Mass of copper (Cu) = 32.8 grams
Molar mass of zinc (Zn) = 65.38 g/mol
Molar mass of copper (Cu) = 63.55 g/mol
Number of moles of zinc (Zn) = Mass of zinc / Molar mass of zinc
Number of moles of zinc (Zn) = 18.0 g / 65.38 g/mol
≈ 0.2755 moles
Number of moles of copper (Cu) = Mass of copper / Molar mass of copper
Number of moles of copper (Cu) = 32.8 g / 63.55 g/mol
≈ 0.5161 moles
Determine the limiting reactant.
The mole ratio between zinc and copper in antique brass is 30.0% zinc and 70.0% copper, which is approximately a 3:7 ratio.
Zn : Cu = 0.2755 moles : 0.5161 moles
≈ 1 : 1.87
Since the mole ratio between zinc and copper is approximately 1:1.87, the copper is the limiting reactant as it is closer to the 70% required for antique brass.
Calculate the mass of antique brass.
To calculate the mass of antique brass, we will use the mass of copper (the limiting reactant) since it determines the maximum amount of product that can be formed.
Mass of antique brass = Mass of copper / Percentage of copper in antique brass
Mass of antique brass = 32.8 g / 0.70
≈ 46.86 grams
Therefore, approximately 46.86 grams of antique brass can be made from 18.0 grams of zinc and 32.8 grams of copper.
To know more about copper here
https://brainly.com/question/13102529
#SPJ3
Do Newton's Laws still apply in space?
Answers
Answer:
Yes, they work in space. I forgot an example, will edit the post when I found it
Hope this helps!
Zoe left her water bottle capped and in her bedroom. She came back some time later to realize that the bottle was “sweating” and left a ring of liquid on her nightstand
Explain thoroughly the science behind why Zoe’s water bottle is sweating
Answers
Answer:
Condensation
Explanation:
Zoe is quite keen to have noticed what we call condensation. Air contains many components, one of those being water vapor. Like how sugar is soluble in water, water can be said to be "soluble" in air. Water will evaporate into the air to a certain extent. The higher the temperature of the air, the more water the air can hold. If the air has more water that it can hold (potentially because of a temperature decrease), the extra water will come out of the air. Zoe's water bottle was cold, and because the air around Zoe's bottle had cooled down, the air can not hold as much water as it could when it was warm, so the air deposited the extra water in the form of liquid water onto the bottle, giving the illusion that her bottle was sweating.
The boundary between cold and warm air masses is called a ____.
A.
front
B.
storm
C.
climate
D.
flood
Answers
Hey can someone help me fill out these blanks

Answers
Answer:
Constructive interference is when two waves interact causing larger waves because the crest will meet a crest or a trough will meet a trough which adds the waves together changing the size of the amplitude.
Destructive interference is when two waves interact causing smaller waves because the crest will meet a trough or a trough will meet a crest which subtracts the waves together changing the size of the amplitude.
I want to make a precipitate from C4 H6 O4Sr and K3 PO4. Is this possible and if so, how do I do this?
Answers
To make the precipitate, you will need to mix solutions of the two compounds together. The easiest way to do this is to dissolve each compound in water separately, and then mix the solutions together.
Here are the steps to follow:
1. Dissolve 1 mole of C4H6O4Sr in 1 liter of water. This will result in a 1 M solution of C4H6O4Sr.
2. Dissolve 1 mole of K3PO4 in 1 liter of water. This will result in a 1 M solution of K3PO4.
3. Mix the two solutions together in a container that will allow you to observe the formation of the precipitate.
4. You should begin to see the formation of a white, cloudy precipitate as the two solutions mix. This is the strontium phosphate.
5. Mix the contents of the container thoroughly and allow it to sit for several minutes to ensure that the reaction is complete.
6. You can then separate the precipitate from the remaining solution using any method that works for you, such as filtration or centrifugation.
Give the mechanism for the reaction:

Answers
The reaction of 2-Bromo-2-Ethyl-3-Methylbutane with methanol is an example of a nucleophilic substitution reaction.
What is the mechanism of the reaction?In this reaction, the methanol molecule acts as a nucleophile and attacks the carbon atom of the bromoalkane, resulting in the displacement of the leaving group (bromine) and the formation of a new carbon-oxygen (C-O) bond.
The reaction mechanism can be described as follows:
Protonation: In the first step, the methanol molecule acts as a base and abstracts a proton from the sulfuric acid catalyst to form the methoxide ion (CH3O-).
Nucleophilic attack: The methoxide ion then attacks the carbon atom of the bromoalkane, which is electrophilic due to the electron-withdrawing effect of the bromine atom. The attack results in the formation of a transition state in which the carbon-bromine bond is weakened and the carbon-oxygen bond is forming.
Elimination: The transition state then collapses to form the product, methylethylmethylcarbinol, with the simultaneous loss of the bromide ion. This step is known as the elimination step and occurs as the newly formed C-O bond is more stable than the weakened C-Br bond.
Learn more about reaction mechanism:https://brainly.com/question/26690612
#SPJ1
Molecules of hydrogen chloride (HCI) are shown in diagram X and diagram Y. Which of the following describes which diagram shows attractive forces, and why?
A Diagram X, because the dashed lines show the attraction between nonmetal atoms that have a similar attraction to electrons.
B
Diagram X, because the dashed lines show that the positive end of one molecule is attracted to the negative end of an adjacent molecule.
С
Diagram Y, because the dashed lines show the attraction between nonmetal atoms that have a similar attraction to electrons.
D
Diagram Y. because the dashed lines show that the positive end of one molecule is attracted to the negative end of an adjacent molecule.
hu
Answers
Since HCl is a polar molecule, there will be attraction between the the positive end of the dipole in one molecule and the negative end of the dipole in another molecule.
Polar molecules are those molecules in which there is a high difference in electronegativity between the bonding atoms. This results in the appearance of partial charges in the molecule. One end of the molecule has a partial negative charge and the other end of the molecule has a partial positive charge.
Since HCl is a polar molecule, there will be attraction between the positive end of one molecule and the negative end of another molecule.
Learn more: https://brainly.com/app/profile/17437791
Zeros between nonzero digits are significant
Answers
Answer:
Explanation:
If a zero is found between significant digits, it is significant
98.1 mL of 5 M potassium hydroxide is mixed with 39.9 mL of 4.5 M Iron (III) acetate resulting in a precipitate of Iron (III) hydroxide. Calculate the theoretical yield in g of iron (III) hydroxide.
Answers
Given:98.1 mL of 5 M potassium hydroxide is mixed with 39.9 mL of 4.5 M Iron (III) acetate resulting in a precipitate of Iron (III) hydroxide.To calculate the theoretical yield in grams of Iron (III) hydroxide, the first step is to balance the chemical equation for the reaction that takes place between potassium hydroxide and iron (III) acetate. 3KOH + Fe(C2H3O2)3 → Fe(OH)3 + 3KC2H3O2The balanced chemical equation for the reaction that takes place between potassium hydroxide and iron (III) acetate can be represented as follows;3KOH + Fe(C2H3O2)3 → Fe(OH)3 + 3KC2H3O2The molar mass of Fe(OH)3 is calculated as follows;Molar mass of Fe(OH)3 = Atomic mass of Fe + (3 x Atomic mass of O) + (3 x Atomic mass of H) = (55.85 g/mol) + (3 x 16 g/mol) + (3 x 1 g/mol) = 106.85 g/molThus the molar mass of Fe(OH)3 is 106.85 g/mol.To determine the theoretical yield of Iron (III) hydroxide we must first determine the limiting reactant (the reactant that is fully consumed in the reaction) among potassium hydroxide and iron (III) acetate.Limiting ReactantIn order to find out the limiting reactant among potassium hydroxide and iron (III) acetate, we will first find out the number of moles of each using the formula;Moles = Concentration x Volume in Liters (L)Moles of KOH = Concentration of KOH × Volume of KOH = 5 M × (98.1 mL/1000 mL) = 0.4905 moles Moles of Fe(C2H3O2)3 = Concentration of Fe(C2H3O2)3 × Volume of Fe(C2H3O2)3 = 4.5 M × (39.9 mL/1000 mL) = 0.17955 molesBased on the balanced chemical equation, the mole ratio of KOH to Fe(C2H3O2)3 is 3:1. Hence, the limiting reactant is Fe(C2H3O2)3 since it is lesser in moles compared to KOH. This means that all of the 0.17955 moles of Fe(C2H3O2)3 will be consumed in the reaction while 0.4905 - (0.17955 x 3) = 0.05145 moles of KOH will be left over after the reaction is complete.The theoretical yield is then calculated using the limiting reactant. We can calculate the number of moles of Fe(OH)3 produced from 0.17955 moles of Fe(C2H3O2)3 using the balanced chemical equation. The mole ratio of Fe(C2H3O2)3 to Fe(OH)3 is 1:1. Hence;Moles of Fe(OH)3 = Moles of Fe(C2H3O2)3 = 0.17955 moles. The mass of Fe(OH)3 is then calculated using the formula;Mass = Number of moles × Molar massMass of Fe(OH)3 = Number of moles of Fe(OH)3 × Molar mass of Fe(OH)3 = 0.17955 moles × 106.85 g/mol = 19.179 gTherefore, the theoretical yield of Fe(OH)3 is 19.179 g.
The theoretical yield of iron (III) hydroxide is 19.19 grams.
What is the theoretical yield of iron (iii) hydroxide?The balanced chemical equation for the reaction between potassium hydroxide and iron (III) acetate is:
3 KOH + Fe(C₂H₃O₂)₃ → Fe(OH)₃ + 3 KC₂H₃O₂
To calculate the theoretical yield of iron (iii) hydroxide, first, we determine the limiting reagent.
The number of moles of each reactant:
Number of moles (n) = Molarity (M) × Volume (V)
For potassium hydroxide (KOH):
n(KOH) = 5 M × 0.0981 L
number of moles = 0.4905 moles
For iron (III) acetate (Fe(C₂H₃O₂)₃):
number of moles = 4.5 M × 0.0399 L
number of moles = 0.17955 moles
Since the stoichiometric ratio is 1:1, the number of moles of Fe(OH)₃ = 0.17955 moles.
The molar mass of Fe(OH)₃ = 106.88 g/mol
Theoretical yield = Number of moles × Molar mass
Theoretical yield = 0.17955 moles × 106.88 g/mol
Theoretical yield= 19.19 grams
Learn more about theoretical yield at: https://brainly.com/question/25996347
#SPJ1