Mass = 35g
Density = 5 g/cm3
∙What is the Volume?
Answers
Answer:
7cm3
.........
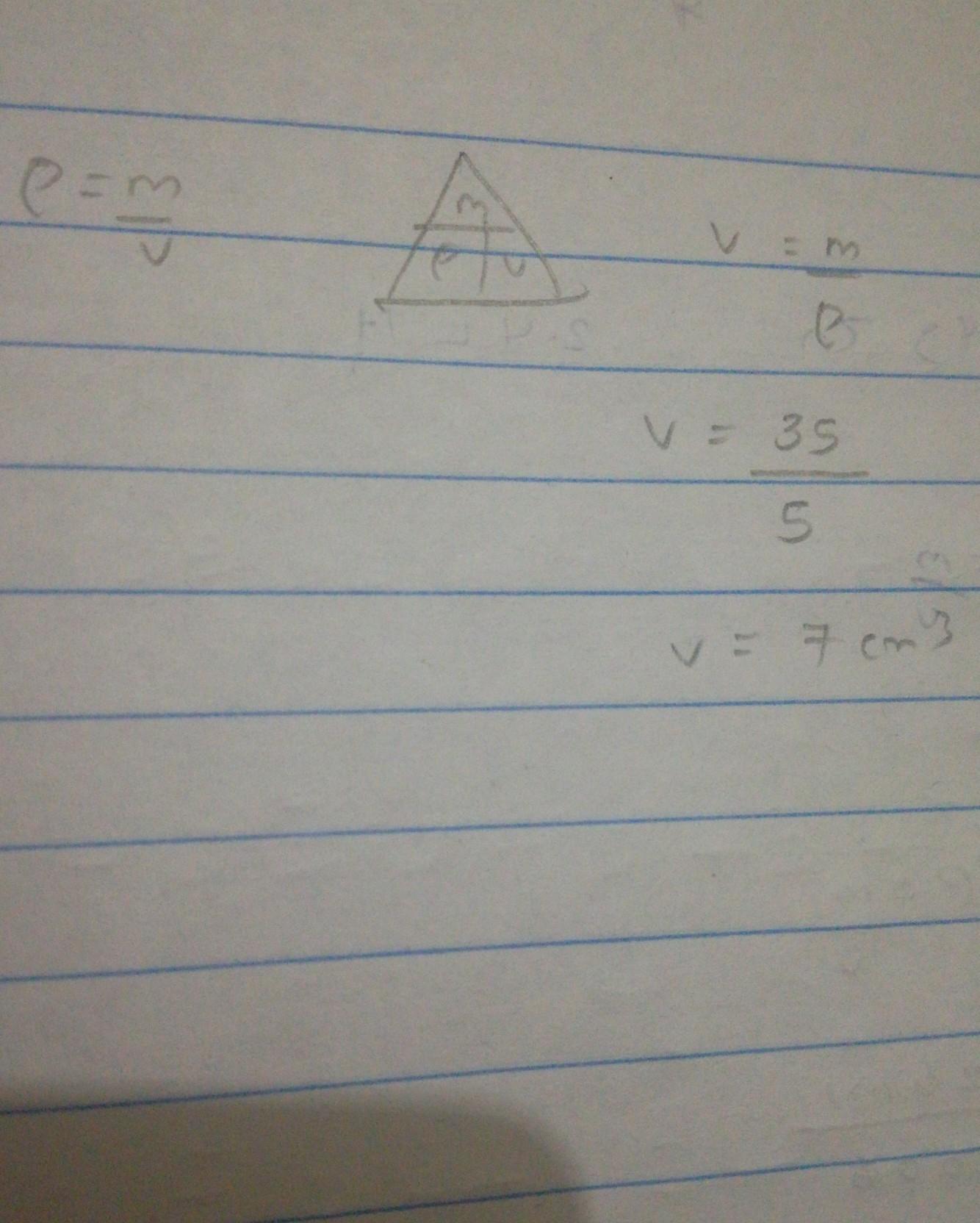
Related Questions
If 4.46 grams of sodium hydroxide reactions with aluminum. How
many grams of aluminum hydroxide was produced?
Answers
The mass (in grams) of aluminum hydroxide produced when 4.46 g of sodium hydroxide reacts is 2.90 grams
How do I determine the mass of aluminum hdroxide produced?First, we shall obtain the balanced equation for the reaction. This is given below
3NaOH + Al → 3Na + Al(OH)₃
Molar mass of NaOH = 40 g/molMass of NaOH from the balanced equation = 3 × 40 = 120 g Molar mass of Al(OH)₃ = 78 g/molMass of Al(OH)₃ from the balanced equation = 1 × 78 = 78 gFrom the balanced equation above,
120 g of NaOH reacted to produce 78 g Al(OH)₃
With the above information, we shall determine the mass of Al(OH)₃ produced from 4.46 grams of NaOH. Details below:
From the balanced equation above,
120 g of NaOH reacted to produce 78 g Al(OH)₃
Therefore,
4.46 g of NaOH will react to produce = (4.46 × 78) / 120 = 2.90 g of Al(OH)₃
Thus, the mass of aluminum hydroxide, Al(OH)₃ produced is 2.90 g
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
The molar mass of barium nitrate (Ba(NO3)2) is 261. 35 g/mol. What is the mass of 5. 30 × 1022 formula units of Ba(NO3)2? 0. 0900 g 12. 0 g 23. 0 g 3,130 g.
Answers
Answer:
\(\boxed{\boxed {\sf 23.0 \ g \ Ba(NO_3)_2}}\)
Explanation:
We are asked to find the mass of 5.30 ×10²² formula units of barium nitrate.
1. Formula Units to MolesFirst, we convert formula units to moles using Avogadro's Number or 6.022×10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are formula units of barium nitrate.
Set up a conversion factor using Avogadro's Number.
\(\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
We are converting 5.30×10²² formula units, so we multiply by this value.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
Flip the conversion factor so the units of formula units of barium nitrate cancel.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}\)
\(5.30 \times 10^{22} *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23}}\)
\(\frac{5.30 \times 10^{22} }{6.022 \times 10^{23}} \ mol \ Ba(NO_3)_2\)
\(0.0880106276984 \ mol \ Ba(NO_3)_2\)
2. Moles to GramsNext, convert moles to grams using the molar mass. The molar mass of barium nitrate is 261.35 grams per mole.
Set up a conversion factor using the molar mass.
\(\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
Multiply by the number of moles we calculated.
\(0.0880106276984 \ mol \ Ba(NO_3)_2 *\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
The units of moles of barium nitrate cancel.
\(0.0880106276984*\frac{ 261.35 \ g \ Ba(NO_3)_2} {1}\)
\(23.001577549 \ g \ Ba(NO_3)_2\)
If this is rounded to the tenths place, the 0 in the hundredth place tells us to leave the 0 in the tenths place.
\(23.0 \ g \ Ba(NO_3)_2\)
The mass of 5.30 ×10²² formula units of barium nitrate is approximately 23.0 grams.
What would happen if our intestines were not coiled (twisted) inside our body?
Answers
Answer:
If there is a change in the natural shape of the intestines, this can be known as a twisted bowel. A twist in the small intestine is referred to as a volvulus. Twists in the large intestine are known as a colonic volvulus. These abnormal twists or loops can cause an obstruction or other medical conditions which could be fatal. If signs of twisting bowel present, it is important to seek medical attention as quickly as possible.
the ionization energy of o2 is 1205 kj/mol. what is the maximum wavelength of light capale of causing the ionization of o2?
Answers
O2 can only be ionized by light with a maximum wavelength of 99.39 nm.
What is ionization energy?The ionization energy measures an element's ability to participate in chemical processes that call for the creation of ions or the donation of electrons.
ionization energy of O2 = 1205 kJ
energy = hv
= h × wavelength/speed of light
wavelength = 1205000× 3 ×10⁸ /6.626 ×10³⁴
wavelength = 99.39 nm.
He is symbolized by the highest ionization energy. The outermost shell has a high ionization energy, is stable, and does not frequently become unstable due to electron loss. Ionization can be induced by waves with energies greater than 134 nm. 225nm light lacks the energy to ionize gold because it is greater than 134nm.
To know more about ionization energy, visit:
https://brainly.com/question/28385102
#SPJ4
How many neutrons are in the nucleus of an atom with a mass number of 80 and an atomic number of 35?
Answers
Answer: 45
Explanation:
mass number = number of protons and neutrons
atomic number = number of protons
to find neutrons subtract atomic number from mass number
so 80 - 35 = 45 neutrons
1.How many grams of zinc Zn and oxygen O2 will be needed to form 324 grams of ZnO after the reaction?
Answers
Answer: pretty sure its 0.214 moles * 40.3g/mole = 8.6g (2 sig figs) of MgO, and
0.035 moles * 40.3g/mole = 1.4g of MgO
Explanation:
Deciding that she was thirsty, Ms. Smith decided to mix up some lemonade using 2 tablespoons of lemonade, and 2 cups of water. What is the solute and solvent?
Answers
Explanation:
solute is the substance being dissolved so that is the 2 tablespoons of lemonade and the 2 cups of water is the solvent.
if m&m are added to the periodic table as a "new" element what would their atomic mass be? a standard bag of m&ms = 1.75 oz and contains about 55 m&ms. (16oz = 453.6 g) put answer in (g/mol)
Answers
Answer: 5.43x10^23 g/mole M&M's
Explanation:
1 bag of M&M's = 1.75oz (1.75oz)*(453.6g/16oz) = 49.61 g
1 bag = 55 M&M's
(49.61 g)/55 M&M = 0.9021 g/MM
1 mole M&M's = 6.023x10^23 M&M's
(6.023x10^23 M&M's)*(0.9021 g/MM's) = 5.43x10^23 g/mole molar mass
What is a radio wave?
Answers
Answer:
Radio waves are a type of electromagnetic radiation best-known for their use in communication technologies, such as television, mobile phones and radios.
Given the reaction: 2na + 2h2o → 2na+ + 2oh− + h2
which substance is oxidized?
1.

h2
2.

h+
3.

na
4.

na+
Answers
Answer:
\(\large \boxed{\text{3. Na}}\)
Explanation:
We can use oxidation numbers to decide which substance is reduced.
\(\rm 2\stackrel{\hbox{0}}{\hbox{Na}} + 2\stackrel{\hbox{+1}}{\hbox{ H}_{2}}\stackrel{\hbox{-2}}{\hbox{O}}\longrightarrow \rm 2\stackrel{\hbox{+1}}{\hbox{Na}^{+}} + 2\stackrel{\hbox{-2}}{\hbox{O}}\stackrel{\hbox{+1}}{\hbox{H}^{-}} + \stackrel{\hbox{0}}{\hbox{H}_{2}}\)
The oxidation number of Na changes from 0 in Na to +1 in Na⁺.
The oxidation number of H changes from +1 in H₂O to 0 in H₂.
\(\text{An increase in oxidation number is oxidation, so $\large \boxed{\textbf{Na}}$ is the substance oxidized.}\)
1 and 4 are wrong because H₂ and Na⁺ are products.
2. is wrong because there is no H⁺ to be oxidized or reduced.
CO+O2=CO2 balanceo de ecuacion
Answers
Answer:
2CO + O2 = 2CO2
Explanation:
espero que esto ayude
You need to prepare 100.0 mL of a pH 4.00 buffer solution using 0.100M benzoic acid (pK
a
=4.20) and 0.240M sodium benzoatc. How many milliliters of each solution should be mixed to prepare this buffer? benzoic acid:
Previous question
Answers
To prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.The ratio of benzoic acid to sodium benzoate in the buffer solution using the Henderson-Hasselbalch equation.
To prepare a pH 4.00 buffer solution using benzoic acid and sodium benzoate, we need to calculate the appropriate volumes of the 0.100 M benzoic acid and 0.240 M sodium benzoate solutions.
First, we need to determine the ratio of benzoic acid to sodium benzoate in the buffer solution. The Henderson-Hasselbalch equation can help us with this calculation:
pH = pKa + log([A-]/[HA])
Given that the pH is 4.00 and pKa is 4.20, we can rearrange the equation:
log([A-]/[HA]) = pH - pKa
log([A-]/[HA]) = 4.00 - 4.20
log([A-]/[HA]) = -0.20
Next, we take the antilog of -0.20 to find the ratio of [A-] to [HA]:
[A-]/[HA] = antilog(-0.20)
[A-]/[HA] = 0.63
The ratio of [A-] to [HA] is 0.63.
Now, let's calculate the volumes of each solution needed. Let's assume x represents the volume (in mL) of the 0.100 M benzoic acid solution and y represents the volume (in mL) of the 0.240 M sodium benzoate solution.
Since the total volume is 100.0 mL, we have the equation: x + y = 100
Considering the ratio of [A-] to [HA] as 0.63, we can write the equation: y/x = 0.63
Solving these two equations simultaneously will give us the volumes of each solution:
x + y = 100
y/x = 0.63
By substituting y = 0.63x from the second equation into the first equation, we get:
x + 0.63x = 100
1.63x = 100
x = 61.35 mL (rounded to two decimal places)
Substituting this value back into the equation x + y = 100, we find:
61.35 + y = 100
y = 38.65 mL (rounded to two decimal places)
Therefore, to prepare the pH 4.00 buffer solution, you should mix approximately 61.35 mL of the 0.100 M benzoic acid solution with 38.65 mL of the 0.240 M sodium benzoate solution.
To know more about the Henderson-Hasselbalch equation, click here, https://brainly.com/question/31732200
#SPJ11
Match each pollution type with the method to control it.1soil pollution2air pollution3water pollution1gas adsorption2composting3settling
Answers
Explanation:
soil pollution
composting
air pollution
gas absorbtion
water pollution
settling
g a catalyst is added to a system at equilibrium. which statement is true? e9'1j c,o(\c; (a) the temperature will decrease. (b) the equilibrium constant will increase. (c) the concentration of products will decrease. (d) if the system is disturbed, it will return to equilibrium faster.
Answers
If the catalyst is added to a system at equilibrium then the reaction follows an alternative pathway of lower activation energy.
What exactly are catalytic enzymes?Any substance that speeds up a reaction without consuming it first. The catalysts for numerous crucial metabolic events are naturally occurring substances called enzymes.
What is the Catalyst effect on equilibrium?By establishing a novel low-energy pathway for turning reactants into products, a catalyst quickens a reaction.It has no impact on the equilibrium constant but accelerates and reverses reactions that go through the same transition state.The equilibrium composition of a reaction mixture is unaffected.It doesn't appear in the expression for the equilibrium constant or the balanced chemical equation.It reduces the activation energy of both forward and reverses processes by the same amount.Learn more about activation energy here:-
https://brainly.com/question/5280701
#SPJ4
How much of a sample remains after five half-lives have occurred?
1/5 of the original sample
1/25 of the original sample
1/32 of the original gample
1/64 of the original sample
Answers
The first half-life, we have 1 • 1/2 = 1/2 left.
After two: 1/2 • 1/2 = 1/4
Three: 1/4 • 1/2 = 1/8
Four: 1/8 • 1/2 = 1/16
And five: 1/16 • 1/2 = 1/32.
Answer:
1/32
Explanation:
2W + X -> Y+ Z
has the mechanism shown below. What is the rate law for this reaction?

Answers
According to chemical equilibrium,the rate law of the given chemical reaction is rate=K[W]³.
What is chemical equilibrium?Chemical equilibrium is defined as the condition which arises during the course of a reversible chemical reaction with no net change in amount of reactants and products.A reversible chemical reaction is the one wherein the products as soon as they are formed react together to produce back the reactants.
At equilibrium, the two opposing reactions which take place take place at equal rates and there is no net change in amount of the substances which are involved in the chemical reaction.At equilibrium, the reaction is considered to be complete . Conditions which are required for equilibrium are given by quantitative formulation.
Factors which affect chemical equilibrium are change in concentration , change in pressure and temperature and presence of catalyst.As second step s slow step the rate law comes from this step where K=[product]\(^moles\)=[W]³ and concentration of X does not matter as it is present in excess.
Learn more about chemical equilibrium,here:
https://brainly.com/question/4289021
#SPJ9
What is the main cause of global convection currents?
Answers
Answer:
The main cause of global convection currents is the uneven heating of the earth by the sun. Hope this helped! :)
The main cause of global convection current is differential heating, that is the temperature difference in different levels of atmosphere.
What is convection current?Convection current is the heat transfer by fluid movement in the earth crust between different temperature regions. The density of fluids differs due to the temperature gradients and thus heated fluids raises and cooled fluid sinks.
The heated fluid movement causes the movement of plates of earth. The molten rock deep inside the earth crust circulates by the convection current.
Hence, the main cause of global convection current is the differential heating of the atmosphere.
To know more about convection currents, refer the link below:
https://brainly.com/question/9739063
#SPJ2
reactions that generate products enriched in one enantiomer are said to be . multiple choice question.
Answers
Enantioselective are reactions that generate products that are enriched in one enantiomer.
What are enantiomers?Enantiomers are pairs of molecules that exist in two forms that are mirror images of each other but are not superimposable. The enantiomers are chemically identical in all other respects. Enantiomeric pairs are distinguished by the direction in which they rotate polarized light when dissolved in solution, either dextrorotatory (d or +) or levorotatory (l or -). Hence the term optical isomer.
The enantioselectivity of a chromatography system is defined as the preferential interaction of one enantiomer with a chiral selector. It is usually determined as the ratio of the retention factors of the two enantiomers in a chiral chromatography or electrophoresis system.
To know more about enantiomers visit:
https://brainly.com/question/21506956
#SPJ4
the diffusion of water through a selectively permeable membrane is called (osmosis/diffusion).
Answers
The diffusion of water through a selectively permeable membrane is called osmosis.
Osmosis is the movement of water molecules from an area of high concentration to an area of low concentration through a selectively permeable membrane.
A selectively permeable membrane allows some molecules to pass through while blocking others. In the case of osmosis, the membrane allows water molecules to pass through but blocks solute molecules. The movement of water molecules occurs because of the difference in the concentration of solute molecules on either side of the membrane.
The side with a higher concentration of solute molecules attracts water molecules from the other side, causing a net movement of water molecules towards that side until equilibrium is reached. Osmosis is a crucial process for living cells as it helps regulate the balance of water and solutes in the cell.
To learn more about osmosis, click here:
https://brainly.com/question/1799974
#SPJ11
Explain the trend in boiling points as you move down group v11
Answers
Answer:
Fluorine
Chlorine
Bromine
Iodine
Boiling point increases as you go down the group v11
Explanation:
The elements of Group VII are the halogens consisting of f fluorine (F), chlorine (Cl), bromine (Br), iodine (I). All of which are non metals and exists as diatomic molecules - F2, Cl2, Br2, I2 with intermolecular attractions between the two molecules of each element held by Van der Waals dispersion force.
Moving down the group, the size of the atoms increases in size from Fluorine, F2 and Chlorine, Cl2 which are gases to Bromine , Br2 which exists as a liquid to solid, Iodine, I2. This attributes to the increasing in Strength of the Van der Waals forces as you go down the group. In order to break the vanderwaals forces , More heat energy is required to change thier states leading to the increase in boiling point going down the group.
Fluorine
Chlorine
Bromine
Iodine
Boiling point increases as you go down the group
the smallest particle of a compound that still has the compounds properties is called a
A. Molecule
B. Ion
C. Aton
D. Element
Answers
The smallest particle of a compound that still has the compound's properties is called a Molecule.
A molecule is a collection of two or more atoms held together by the attractive forces known as chemical bonds. When speaking of polyatomic ions, the distinction between them and ions is frequently ignored in the fields of quantum physics, organic chemistry, and biochemistry. A molecule can be heteronuclear, which is a chemical compound made up of more than one element, such as water, or homonuclear, which is a molecule made up of atoms of one chemical element, such as the two atoms in the oxygen molecule (O2) (two hydrogen atoms and one oxygen atom; H2O). The term "molecule" is frequently used to refer to any gaseous particle, regardless of its composition, in the kinetic theory of gases.As the noble gases are single atoms, the requirement that a molecule comprise two or more atoms is relaxed. Often, single molecules do not include atoms and complexes joined by non-covalent interactions like hydrogen bonds or ionic bonds.
There are lots of molecules in matter. Most of the oceans and atmosphere are also made up of them. The majority of organic compounds are molecules. Molecules such as proteins, the amino acids that make them up, nucleic acids (DNA and RNA), sugars, carbohydrates, lipids, and vitamins are examples of the constituents of life. The nutrition minerals, such as iron sulphate, are often ionic compounds rather than molecules.
On Earth, however, the vast majority of known solids are composed entirely or mostly of molecules-free crystals or ionic compounds. All the minerals that make up the Earth's composition, as well as sand, clay, pebbles, rocks, boulders, bedrock, the molten interior, and the Earth's core, are among them. Each of these has several chemical linkages, but are not made of identifiable molecules.
For more such questions on molecule , Visit:
https://brainly.com/question/30375112
#SPJ11
An air-to-air heat pump ____ heat from the outside air and deposits it in the conditioned space
Answers
According to the research, the correct option is remove. An air-to-air heat pump removes heat from the outside air and deposits it in the conditioned space.
What are heat pumps?It is characterized by being a thermal machine or a device based on thermodynamics that, through an absorption process, takes heat from one place and transfers it to another, that is, it extracts heat from a cold source to a hot one.
An air-to-air heat pump starts by sucking in outside air or recovers heat from outside and transfers it to a higher temperature level in the ambient air, raising the temperature of the room, being adjustable to adapt to the heating needs of the conditioned space.
Therefore, we can conclude that according to the research, the correct option is remove. An air-to-air heat pump removes heat from the outside air and deposits it in the conditioned space..
Learn more about heat pumps here: https://brainly.com/question/1081420
#SPJ1
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms.

Answers
Answer:
a. pH = 2.22.
b. [H+] = 2.588 x 10⁻⁴ mol/L.
Explanation:
Acids and Bases => Calculating pH of Acids and Bases.
As we saw before, the formulas to find the pH based on the hydrogen ion concentration [H+], and to find the hydrogen ion concentration [H+] based on the pH are the following, respectively:
\(\begin{gathered} pH=-log\lbrack H^+], \\ \\ [H^+]=10^{-pH}. \end{gathered}\)So let's see each case:
a. To find the pH of an H+ concentration of 6.02 x 10⁻³ mol/L we use the pH formula:
\(\begin{gathered} pH=-log\lbrack6.02\cdot10^{-3}], \\ \\ pH=2.220\approx2.22. \end{gathered}\)The answer would be that the pH is 2.22.
b. To find the H+ concentration of a pH of 3.587, we use the [H+] formula:
\(\begin{gathered} \lbrack H{}^+]=10^{-3.587}, \\ \\ [H^+]=2.5882\cdot10^{-4}\text{ mol/L}\approx2.588\cdot10^{-4}\text{ mol/L.} \end{gathered}\)The answer would be that the hydrogen ion concentration [H+] = 2.588 x 10⁻⁴ mol/L.
need help asap first comment gets brainiest answer

Answers

What are three ways fluid flow is important in the food industry?
Answers
hy do you think chemists use the concept of effective nuclear charge? what are some properties that it helps us to understand?
Answers
Effective nuclear charge is really important, because it determines the size and energy of orbitals, which determine most properties of atoms.
Other trends that are important in understanding effective nuclear charge on the periodic table, or z effective trend, are atomic radius, atomic number, and ionization energy. Atomic radius generally increases down a group and decreases across a period.
Effective nuclear charge, Zeff, is a representation of the average electrical field experienced by a single electron. It is the average environmental created by the nucleus and the other e- in the molecule, expressed as a net positive charge at the nucleus.
There are two factors responsible for increasing the effective nuclear charge, which is electrons and protons. The effective nuclear charge, Zeff=Z−S. this value gives information about the charge of an electron.
Learn more about Effective nuclear charge here:- https://brainly.com/question/15874485
#SPJ4
Why is it necessary to mix any solution that does not show an immediate change?.
Answers
Answer:
Explanation:
It is to keep the mixture homogeneous
The aim is to produce a homogenous mixture.
Describe the mixed solutions?In homogeneous solutions, particles of one substance the solute and those of another the solvent are combined, for example, in salty water. Heterogeneous solutions are massive collections (clumps) of the constituent ingredients, such as an oil-in-water emulsion.What do you call a homogenous mixture?A homogeneous mixture is also referred to as a solution. A homogenous combination of two or more components is called a solution. A solute is a substance that dissolves in a medium. The solvent is the substance that a solute dissolves in.What are some homogeneous mixture examples?Examples of homogenous mixtures are as follows:
ocean waterWine.Vinegar.Steel.Brass.Air.Gas, natural.Bloodlearn more about homogenous mixtures here https://brainly.com/question/14441492
#SPJ2
Explain Garri industry
Answers
Answer:
In West Africa, garri refers to the creamy granular flour obtained by processing the starchy tuberous roots of freshly harvested cassava. ... Flour foodstuffs mixed with cold or boiled water are a major part of the diet amongst the various ethnicities of Nigeria, Benin Republic, Togo, Ghana, Guinea, Cameroon and Liberia.
Explanation:
The waves energy runs through water molecules, but they do not move the water molecules in the direction of the waves. Lesson 2.13 Question 24 options: True False
Answers
If 4000 J are needed to melt 8.00 grams of “y” at its melting point what is the heat of fusion of “y”
Answers
The heat of fusion of the solid that melts is 500 J/g.
What is the heat of fusion?When we talk about the heat of fusion then our mind must go to the heat that does not raise the temperature of the object but would cause the solid to be able to melt and we can be able to also put down the formula;
H = mL
m = mass of the solid
L = heat of fusion
H = heat supplied
Then we have;
4000 = 8 L
L = 4000/8
L = 500 J/g
Thus the heat of the fusion of the solid material here is 500 J/g
Learn more about heat of fusion: https://brainly.com/question/14053504
#SPJ1