Answers
The first antipsychotic was chlorpromazine, which was then followed by numerous additional antipsychotics, many of which had different chemical structures, this is the initial method of treating schizophrenia.
The first antipsychotic was chlorpromazine, which was followed by numerous other antipsychotics, many of which had different chemical structures. About a quarter of people who have psychosis only experience one episode. Psychosis is a mental health condition that affects how people perceive or respond to their environment. Other therapies included brain tissue removal (lobotomies). From the 1930s to the 1940s, lobotomies were frequently used to treat schizophrenia, severe anxiety, and depression.
to know more about schizophrenia please visit.
https://brainly.com/question/28345800
#SPJ4
Related Questions
pls help I’ll give allot of points I need all the answers pls anybody help!!!
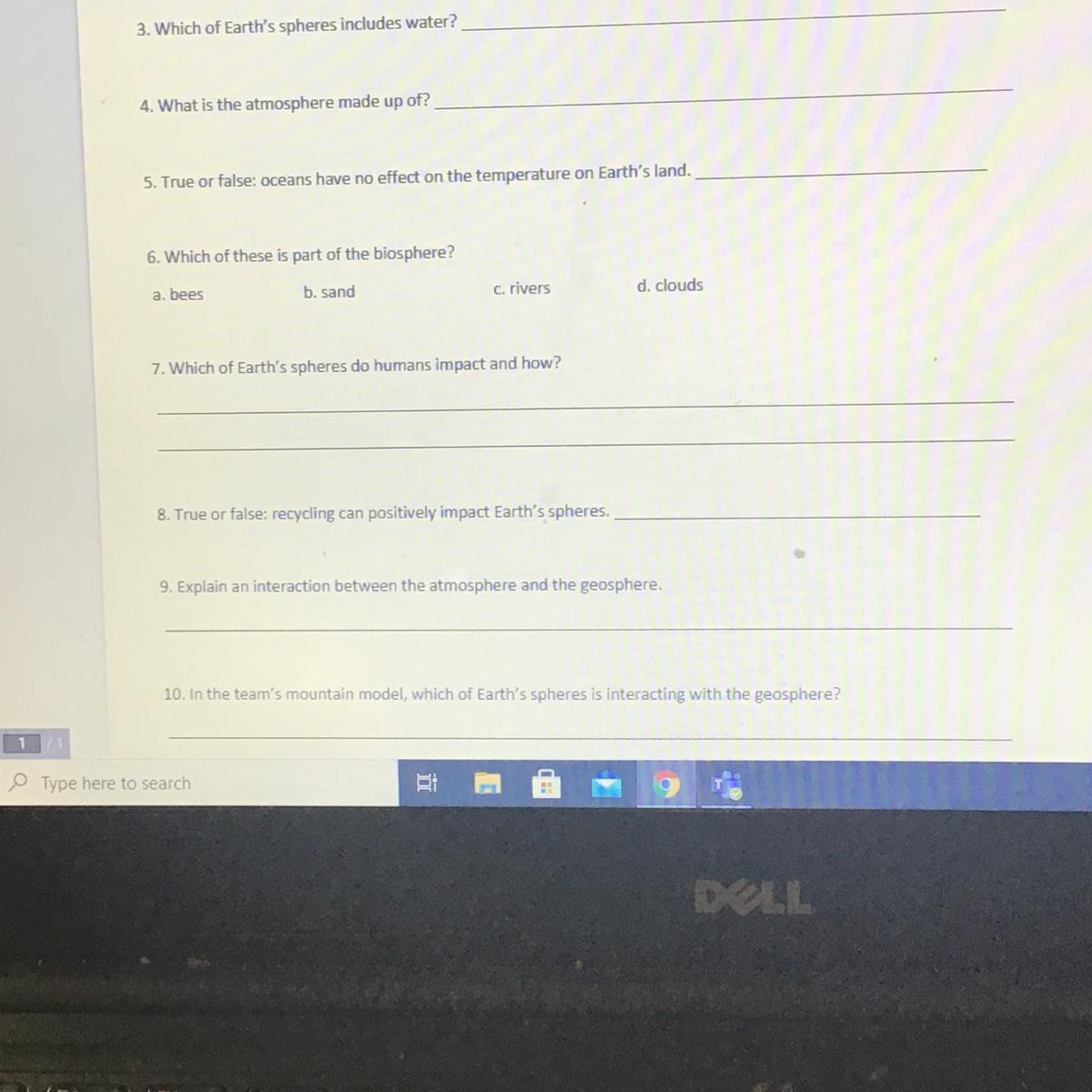
Answers
4: Gases
5: False - I think
6: D - Clouds
SOME PEOPLE PLACE GLOW STICKS IN
THE FREEZER TO MAKE THEM LAST
LONGER. WHY DO YOU THINK THIS
WORKS?
Answers
What is the pH of the solution formed when 12.50 mL of 1.05 M KOH is added to 50.0 mL of 0.225 M HBr?
A. 0.65
B. 1.52
C. 12.48
D. 13.35
Answers
Answer: D
Explanation:
When 12.50 mL of 1.05 M KOH is added to 50.0 mL of 0.225 M HBr, the resulting solution has a pH of 13.35.
Here’s how to calculate it:
First, we need to determine the number of moles of KOH and HBr in the solution:
moles of KOH = (12.50 mL) * (1.05 mol/L) * (1 L/1000 mL) = 0.013125 mol moles of HBr = (50.0 mL) * (0.225 mol/L) * (1 L/1000 mL) = 0.01125 mol
KOH is a strong base and HBr is a strong acid, so they will react completely to form water and a salt (KBr):
KOH + HBr -> KBr + H2O
The number of moles of KOH is greater than the number of moles of HBr, so there will be an excess of KOH in the solution after the reaction is complete:
moles of excess KOH = moles of KOH - moles of HBr = 0.013125 mol - 0.01125 mol = 0.001875 mol
The total volume of the solution is the sum of the volumes of KOH and HBr:
total volume = 12.50 mL + 50.0 mL = 62.5 mL
The concentration of excess OH- ions in the solution is:
[OH-] = moles of excess KOH / total volume = 0.001875 mol / (62.5 mL * (1 L/1000 mL)) = 0.03 M
The pOH of the solution can be calculated using the formula pOH = -log[OH-]:
pOH = -log(0.03) = 1.52
The pH can be calculated using the formula pH + pOH = 14:
pH = 14 - pOH = 14 - 1.52 = 13.35
So the correct answer is D. 13.35.
Please help me with this
If somebody posts b.u.l.l.s.h.i.t. answers, please report them!!
Answers
Answer:
where is the question
Explanation:
the work function of magnesium metal is 5 86/10J
a, calculate the minimum frequency of required to release elections from the metal.
b, calculate the kinetic energy of the ejected electronic light of frequency 2.00/10 s is used to irradiating the metal.
Answers
a) To calculate the minimum frequency of electromagnetic radiation required to release electrons from the metal, you can use the following formula:
f = W / h
where f is the minimum frequency of electromagnetic radiation required, W is the work function of the metal in joules, and h is the Planck constant in joules per second.
Plugging in the values for W and h, you get:
f = (5.86 x 10^-19 J) / (6.626 x 10^-34 J/s) = 8.9 x 10^14 Hz
This is the minimum frequency of electromagnetic radiation required to release electrons from the magnesium metal.
b) To calculate the kinetic energy of the ejected electronic light of frequency 2.00 x 10^14 Hz, you can use the following formula:
KE = hf - W
where KE is the kinetic energy of the ejected electron, h is the Planck constant in joules per second, f is the frequency of the electromagnetic radiation in hertz, and W is the work function of the metal in joules.
Plugging in the values for h, f, and W, you get:
KE = (6.626 x 10^-34 J/s) * (2.00 x 10^14 Hz) - (5.86 x 10^-19 J) = 1.32 x 10^-19 J - 5.86 x 10^-19 J = -4.54 x 10^-20 J
This is the kinetic energy of the ejected electron when light of frequency 2.00 x 10^14 Hz is used to irradiate the magnesium metal. Since the kinetic energy is negative, this means that the electron is not released from the metal when irradiated with this frequency. The frequency of the electromagnetic radiation needs to be higher than the minimum frequency required to release the electron in order for the electron to be ejected from the metal.
11. In a reaction from number 10, 65.0g of Ni(NO3)2 is reacted with 58.0g KOH. Which is
the limiting reactant? Show your work for credit. (4pts)
Answers
Answer:
Ni(NO3)2 is the limiting reactant.
Explanation:
- First, we balance the equation...
Ni(NO3)2 + 2 KOH ---> 2 KNO3 + Ni(OH)2
- Second, we find the moles of each substance...
65g Ni(NO3)2 / 182.703g Ni(NO3)2 = 0.356 mol Ni(NO3)2
58g KOH / 56.1056g KOH = 1.034 mol KOH
- Third, to make the molar ratio equal to each other for comparison, we either multiply KOH by 1/2 or multiply Ni(NO3)2 by 2 to compare the number of moles; because the Ni(NO3)2 to KOH molar ratio is 1 to 2. Note that the multiplication of moles is only for comparison. We do not use these multiplied values. We use the values from step 2...
0.356 mol Ni(NO3)2 * 2 = 0.712 mol Ni(NO3)2
0.712 mol Ni(NO3)2 < 1.034 mol KOH ... Ni(NO3)2 is the limiting reactant.
A compound is found to contain 9.227 % boron and 90.77 % chlorine by mass. What is the empirical formula for this compound?
Answers
Assuming a 100 g sample of the compound, we can convert the mass percentages to masses in grams:
- 9.227 g B
- 90.77 g Cl
Next, we need to convert these masses to moles using the atomic masses of the elements:
- B: 10.81 g/mol
- Cl: 35.45 g/mol
- 9.227 g B ÷ 10.81 g/mol = 0.853 mol B
- 90.77 g Cl ÷ 35.45 g/mol = 2.562 mol Cl
Now we need to divide both mole values by the smaller of the two, which is 0.853 mol:
- 0.853 mol B ÷ 0.853 mol = 1.000 mol B
- 2.562 mol Cl ÷ 0.853 mol = 3.000 mol Cl
This gives us a B:Cl ratio of 1:3. The empirical formula for the compound is therefore BCl3.
Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Explanation:
Mass of boron and chlorine is 9.224% and 90.74%
Calculate the density of air at exactly 35°C and 1.00 atm (assuming that air is 80.0 percent N2 and 20.0 percent O2).
Answers
The density of air at exactly 35°C and 1.00 atm assuming that air is 80.0 percent N₂ and 20.0 percent O₂ is 1.139 g/L
How do I determine the density of the air?We'll begin by obtaining the average atomic mass of the air. This can be obtained as follow:
Molar mass of N₂ = 28 g/molAbundance of N₂ = 80%Molar mass of O₂ = 32 g/molAverage atomic mass of air =?Average atomic mass of air = (Molar mass of N₂ × abundance) + (Molar mass of O₂ × abundance)
Average atomic mass of air = (28 × 80%) + (32 × 20%)
Average atomic mass of air = 28.8 amu
Finally, we shall determine the density of the air. This is illustrated below:
Temperature (T) = 35 °C = 35 + 273 = 308 KPressure (P) = 1 atmGas constant (R) = 0.0821 atm.L/Kmol Average atomic mass of air (M) = 28.8 amuDensity =?Density = PM / RT
Density = (1 × 28.8) / ( 0.0821 × 308)
Density = 1.139 g/L
Thus, the density of the air is 1.139 g/L
Learn more about density:
https://brainly.com/question/952755
#SPJ1
100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch
Calculate the g and mL necessary to make this solution
Answers
52.6g and 23.8mL necessary 100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch to make this solution.
What is malonic acid?
The chemical formula of malonic acid is CH2(COOH)2. Malonates include the ionized form of malonic acid as well as its esters and salts. Because it interferes with respiration, malonic acid is extremely harmful, especially in cases of cancer and other degenerative disorders (the making of ATP in mitochondria). Malonic acid is a somewhat unstable substance with limited practical uses. Beetroot contains its calcium salt, however the acid itself is often made by hydrolyzing diethyl malonate.
To learn more about malonic acid, refer: -
https://brainly.in/question/46046349
SPJ1
How many liters would you need to make a 1 m solution if you have 6 mol of sodium hydroxide.
Answers
The liters would we need to make the 1 M solution if we have 6 mol of sodium hydroxide of 6 L.
The moles of the sodium hydroxide = 6 mol
The molarity of the sodium hydroxide = 1 M
The expression for the molarity is as follows :
The molarity = moles / volume in L
The volume of the sodium hydroxide = moles / molarity
The volume of the sodium hydroxide = 6 / 1
The volume of the sodium hydroxide = 6 L.
Thus the volume of the sodium hydroxide is 6L in the 1 M of the solution.
To learn more about moles here
https://brainly.com/question/1097767
#SPJ4
2.Draw a structural formula for 2,3-octene (C8H16) which has a double bond between the second and third carbon in the chain. According to our lessons, what class of compound is it? How do you know?
Answers
First, we have to draw eight carbons and a double bond between carbon two and carbon three, always complying with bonding the carbon four times:
The double bond is between carbon 2 and 3 (the number indicates it in red) and its color is blue. This class of compound is called: alkene because an alkene is a hydrocarbon with one or more carbon-carbon double covalent bonds.

A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO3 → Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3.
After decomposition, the Na2CO3 had a mass of 1.57 grams.
A. Determine the mass of the H2CO3 produced.
B. Calculate the percentage yield of H2CO3 for the reaction. Show your work or
describe the calculation process in detail.
Answers
Answer:
Explanation:
a) The mass of the reactants is 2.36 grams, and the mass of the products is 1.57 grams plus the mass of the carbonic acid. Thus, using the law of conservation of mass, we get the mass of the carbonic acid is 2.36 - 1.57 = 0.79 grams.
b) The gram-formula mass of sodium bicarbonate is 84.006 g/mol, meaning that 2.36/84.006 = 0.028 moles were consumed. Thus, this means that in theory, 0.014 moles of carbonic acid should have been produced, which would have a mass of (0.014)(62.024)=0.868 grams. Thus, the percentage yield is (0.79)/(0.868) * 100 = 91%
At 20C what is the molar mass of a gas with a denisty of 1.02g/L at 2.13atm
Answers
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.The molar mass of an element or compound is the mass of one mole of that substance. A mole is the SI unit for the amount of a substance.
It's defined as the amount of a substance that contains the same number of entities as there are atoms in 12 grams of carbon-12.Molar mass (M) = mass (m) ÷ amount of substance (n)So, M = m/n
Where m is the mass in grams and n is the number of moles. The unit of molar mass is grams per mole (g/mol).
The ideal gas law is used to calculate the molar mass of a gas. The ideal gas law is:P V = n R T,Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Convert the density to grams per liter: 1.02 g/L.
The density is mass/volume, thus 1.02 g/L means that 1 liter of the gas weighs 1.02 g.
This means that 1 mole of gas will occupy 22.4 L (at standard temperature and pressure, STP).Calculate the number of moles of gas using PV = nRT.P = 2.13 atmV = 22.4 L (at STP)R = 0.0821 L·atm/K·molT = 273.15 K + 20 K = 293.15 K
Thus, n = PV/RT = (2.13 atm × 22.4 L)/(0.0821 L·atm/K·mol × 293.15 K) = 0.973 mol
Calculate the molar mass (M) using M = m/n.m = density × volume = 1.02 g/L × 22.4 L = 22.848 gM = m/n = 22.848 g/0.973 mol = 23.5 g/mol Convert to units of grams per mole: 23.5 g/mol
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.
For more question on compound
https://brainly.com/question/12651906
#SPJ8
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
Which option contains an example of a polyatomic ión? (1 point)
O KNO3
O CaCl₂
O 02
O MgCl
Answers
Answer:
KNO3 is an example of a polyatomic ion
Rank in order of acidity (1= most acidic)

Answers
The rank order of acidity is para nitro phenol > meta nitro phenol > phenol > Para amino phenol.
What does acidity order mean?'The nitro group is a powerful electron withdrawing group. As a result, by removing electron density from the carboxylate ion, it increases the acidity of benzoic acid. This effect is strongest when the nitro group is in the ortho position (the effect is known as ortho effect).
The chemical structure of an acid can be used to predict its relative strength. An acid is generally stronger when the H-A bond is more polar. When the H-A bond is weaker and the conjugate base, A, is more stable, acidity increases.
Thus, The rank order of acidity is para nitro phenol > meta nitro phenol > phenol > Para amino phenol.
To learn more about acidity order, follow the link;
https://brainly.com/question/9423359
#SPJ1
At 1 atm,
how much energy is required to heat 93.0 g H2O(s)
at −10.0 ∘C
to H2O(g)
at 121.0 ∘C?
Use the heat transfer constants found in this table.
Answers
The total energy required to heat the water to the final temperature is 290,550.6 J.
What is the total energy required to heat the water?
The total energy required to heat the water to the final temperature is calculated as follows;
E = Q₁ + Q₂ + Q₃ + Q₄
E = mcΔθ + mf + mcΔθ₂ + mLv
where;
c is specific heat capacity = 4.2 J/gCLv is latent heat of vaporization = 2240 J/gf is heat of fusion of ice = 334 J/gThe heat capacity of the water is calculated as;
E = 93 x 4.2 x (10) + 334 x 93 + 93 x 4.2 x (121 - 0) + 2240 x 93
E = 290,550.6 J
Learn more about heat capacity here: https://brainly.com/question/16559442
#SPJ1
Balance the following reaction. A coefficient of "1" is understood. Choose option "blank" for the correct answer if the
coefficient is "1".
C₂H6+
02-
CO₂ +
✓ H₂O
Answers
Answer:
C₂H6 + O2 → 2CO2 + 3H2O
How much heat must be added to a 34.2 g sample of aluminum in order to raise the temperature of the aluminum 34 oC? (The specific heat of Aluminum is 0.9 J/g oC)
Answers
The amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical property that determines the direction of heat flow between two objects or systems in contact with each other. Temperature is measured in degrees Celsius (°C) or Fahrenheit (°F), or in kelvin (K) in the International System of Units (SI).
The amount of heat (q) required to raise the temperature of a substance can be calculated using the formula:
q = m x c x ΔT
Where:
m = mass of the substance (in grams)
c = specific heat of the substance (in J/g oC)
ΔT = change in temperature (in oC)
Plugging in the values given:
m = 34.2 g
c = 0.9 J/g oC
ΔT = 34 oC
q = (34.2 g) x (0.9 J/g oC) x (34 oC)
q = 1043.52 J
Therefore, the amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
Learn more about Temperature
brainly.com/question/26866637
#SPJ1
Are all covalent molecules soluble in water ?
Answers
Explanation:
determining which kind of elements are soluble, especially covalent molecules. Chemical balances, no matter in bonds. chemical balances, physical balances are reasons why.
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
Calculate the molality when 35. 0 grams of mgcl2 is dissolved in 200. 0 g of solvent.
Answers
1.84mol/Kg
To find the moles of MgCl2;
Number of moles of MgCl2 = (mass of MgCl2) / (molar mass of MgCl2)
Number of moles of MgCl2 = 35.0 g / 95.21 g/mol
Number of moles of MgCl2 = 0.367 moles
We then convert mass of solvent in Kg.
Mass of solvent = 200.0 g
Mass of solvent = 0.200 kg
We can now calculate the molality:
Molality = (moles of solute) / (mass of solvent in kg)
Molality = 0.367 mol / 0.200 kg
Molality = 1.84 mol/kg
Therefore, the molality of the solution is 1.84 mol/kg.
To know more click on:-
https://brainly.com/question/30922107
Which part of the landscape shown in this image is the steepest?

Answers
Answer: A I believe
Explanation:
What are the derived SI units?
Answers
balanced equation in which K2Cr2O7 oxidizes KI in the presence of H2SO4
Answers
The balanced equation \(K_{2}Cr_{2}O_{7}\) oxidizes KI in the presence of \(H_{2}SO_{4}\)can be represented as
6 KI + \(K_{2}Cr_{2}O_{7}\) + 7 \(H_{2}SO_{4}\) → 4 \(K_{2}Cr_{2}O_{7}\) + \(Cr_{2}(SO_{4})_{3}\) + 3 \(I_{2}\)\(I_{2}\) + 7 \(H_{2}O\)
The balanced equation for the oxidation of KI (potassium iodide) by \(K_{2}Cr_{2}O_{7}\)(potassium dichromate) in the presence of \(H_{2}SO_{4}\)(sulfuric acid) can be represented as follows:
6 KI + \(K_{2}Cr_{2}O_{7}\) + 7 \(H_{2}SO_{4}\) → 4 \(K_{2}Cr_{2}O_{7}\) + \(Cr_{2}(SO_{4})_{3}\) + 3 \(I_{2}\)\(I_{2}\) + 7 \(H_{2}O\)
In this equation, \(K_{2}Cr_{2}O_{7}\) is the oxidizing agent, and KI is the substance being oxidized. The sulfuric acid (\(H_{2}SO_{4}\)) serves as a catalyst and provides the necessary acidic conditions for the reaction to occur.
The products of the reaction are potassium sulfate (\(K_{2}SO_{4}\)), chromium(III) sulfate (\(Cr_{2}(SO_{4})_{3}\)), iodine (), and water (\(H_{2}O\)).
Note that this equation represents a stoichiometrically balanced equation, ensuring that the number of atoms of each element is the same on both sides of the equation.
Know more about oxidizes here:
https://brainly.com/question/25886015
#SPJ8
Select the correct answer.
What is the molecularity of this elementary step?
A+ A+B-->C+D
A.
unimolecular
B.
bimolecular
c.
trimolecular
D.
tetramolecular
Answers
Answer: C not really sure thoo
when are larger atoms least likely to be reactive
Answers
Answer:
when they have eight valence electrons.
Explanation:
When atoms do not have a full valence shell, the atoms are more likely to react with other and vice versa.
Answer:
When they have a full shell of electrons
Explanation:
and or eight valence electrons
Write the orbital notation for the following elements

Answers
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8