n2 h2 nh3 if 39.5 mols of nitrogen react with 19.9 mols of hydrogen, how many mols of ammonia will be produced?
Answers
59.4 moles of ammonia will be produced. The molar masses of ammonia is 17 g/mol.
What is ammonia ?Ammonia is corrosive and has alkaline characteristics. Ammonium hydroxide, a caustic solution and weak base, is produced when ammonia gas readily dissolves in water. Ammonia gas compresses easily and turns into a clear liquid when put under pressure. Ammonia is typically transported in steel containers as a compressed liquid.
Due to its slower conversion to nitrate, anhydrous ammonia is the chosen N fertiliser for an autumn application. Spreading out the effort and reducing soil compaction are two benefits of applying N in the fall.
In contrast to many burns, anhydrous ammonia burns are treated by washing the chemical off the skin with water.
Number of moles of nitrogen = 39.5
Number of moles of hydrogen = 19.9 moles
Number of moles of ammonia = 19.9 moles h2 + 39.5 mols = 59.4 moles
To learn more about ammonia refer :
https://brainly.com/question/14854495
#SPJ4
Related Questions
Consider the chemical equation. 2h2 o2 right arrow. 2h2o what is the percent yield of h2o if 87.0 g of h2o is produced by combining 95.0 g of o2 and 11.0 g of h2? use percent yield equals startfraction actual yield over theoretical yield endfraction times 100.. 56.5% 59.0% 88.5% 99.7%
Answers
The percent yield of H₂O, if 87.0 g of H₂O is produced by combining 95.0 g of O₂ and 11.0 g of H₂ is 87.87%.
How do we calculate mass from moles?Mass of any substance will be calculated by using their moles as:
n = W/M, where
W = given or required mass
M = molar mass
Moles of 95g of Oxygen (O₂) = 95g / 32g/mol = 2.96 moles
Moles of 11g of hydrogen (H₂) = 11g / 2g/mol = 5.5 moles
Given chemical reaction is:
2H₂ + O₂ → 2H₂O
From the stoichiometry of the reaction, it is clear that:
1 moles of O₂ = reacts with 2 moles of H₂
2.96 moles of O₂ = reacts with 2×2.96=5.92 moles of H₂
Here hydrogen is the limiting reagent as it has lower moles and formation of water depends on this only.
2 moles of H₂ = produces 2 moles of water
5.5 moles of H₂ = produces 5.5 moles of water
Mass of 5.5 moles of water will be calculated as:
W = (5.5mol)(18g/mol) = 99g
Given theoretical yield of water = 87g
% yield of water will be calculated as:
% yield = (87 / 99)×100 = 87.87%
Hence required value is 87.87%.
To know more about % yield, visit the below link:
https://brainly.com/question/25996347
Answer:
D
Explanation:
If potassium is a soft reactive metal at room temperature, name one other element you would expect to also be a soft reactive metal at room temperature? How do you know?
Answers
Explanation:
Lithium
Sodium
group one elements are highly reactive
Structures and Forces - What is force and external forces.
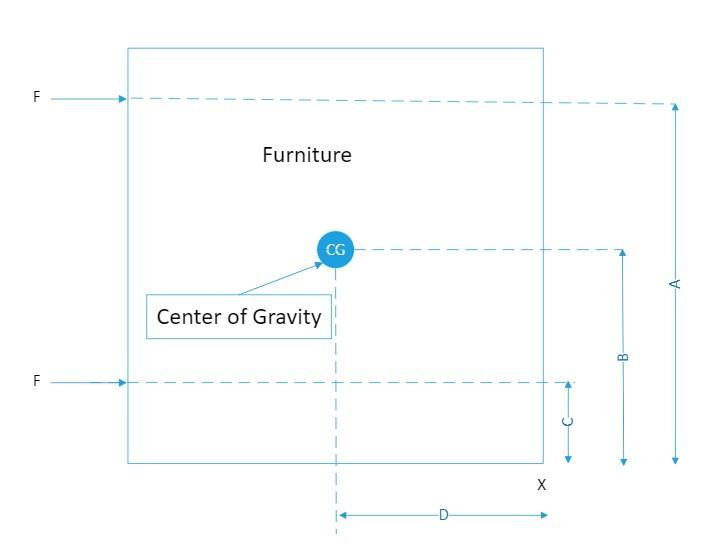
Question 1) Jacob and Ravleen are moving furniture. They need to move the filing cabinet over 6 feet and it is very heavy. Jacob wants to apply force and push it by putting his hands on point A because he thinks it would be easier to move it from the top as there is less weight on the top. Ravleen says it's better to push at point B because the center of gravity is lower. Which person is correct and EXPLAIN why and what may happen if it is pushed in the wrong spot.
Answers
Answer:
The restoring force that prevents the furniture from tipping over when pushing the furniture towards the right, F= W × D
Where;
W = The weight of the furniture
D = The horizontal distance from the center of gravity of the furniture, CG and the to the lower right corner of the furniture
Given that A > D, for the furniture to tip over when pushing above the CG, we have;
F1 × A ≤ W × D
∴ F1 << W or for a force much smaller than W, the weight of the furniture applied above the center of gravity, the furniture will tip over
However when the force F2 is applied, where C < D, we have for no tipping over;
F2 × C ≤ W × D, therefore, given that C < D, when a force much higher than the weight, W, is applied at F2, the furniture will remain upright
Therefore, it is better push at a point lower than or equal to the center of gravity, whereby the point B is lower than the center of gravity, it is better to push at point B, Ravleen is correct and it is better to push at point B because the center of gravity is lower
If the furniture is pushed at the wrong spot it may tip over
Please see attached drawing
Explanation:
understand polarity and how it impacts bonding between water molecules in the liquid and solid.
T/F
Answers
True. Polarity impacts bonding between water molecules in both liquid and solid states. Polarity arises due to the unequal sharing of electrons between oxygen and hydrogen atoms in a water molecule, leading to partial positive and negative charges.
These charges cause water molecules to form hydrogen bonds, which give water its unique properties in liquid and solid states. Water molecules have a polar covalent bond due to the unequal sharing of electrons between the hydrogen and oxygen atoms. This polarity allows water molecules to form hydrogen bonds with each other. In the liquid state, water molecules are constantly moving and forming and breaking hydrogen bonds with each other. In the solid state, the water molecules are arranged in a crystal lattice structure where each molecule is bonded to four others through hydrogen bonds. This arrangement gives ice a lower density than liquid water and allows it to float. The polarity of water also makes it a good solvent for polar and charged substances.
Learn more about Polarity here :-
https://brainly.com/question/1946554
#SPJ11
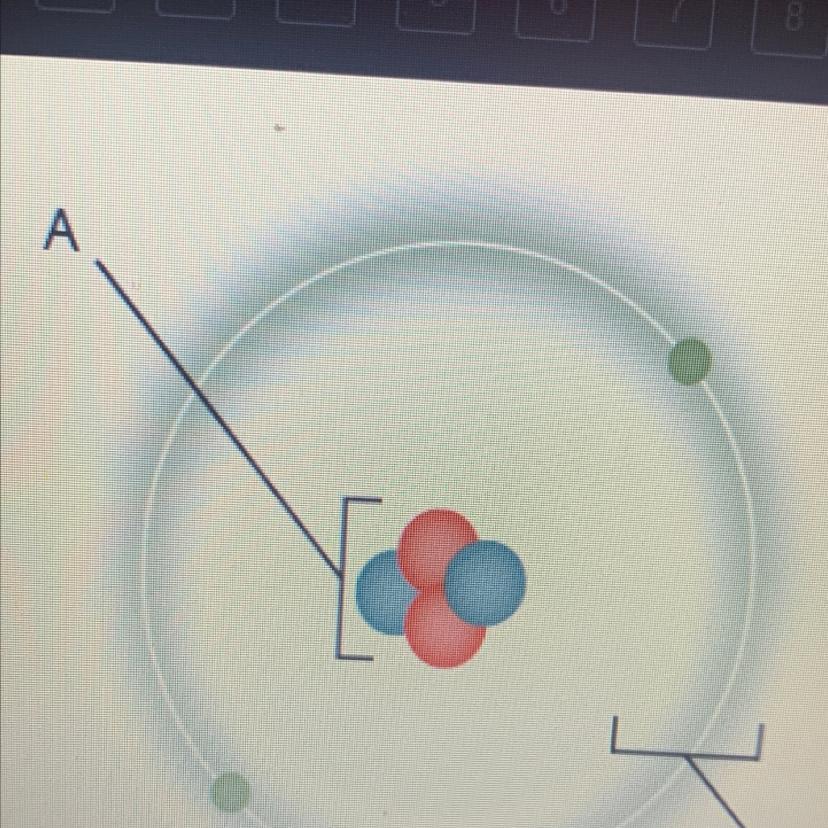
Which is a characteristic of the part of the atom marked “A”?
•it contains most of the mass
•it is negatively charged
•it is mostly empty space
•it is composed of electrons
Answers
Answer:
it contains most of the mass
Some help on these questions would be awesome:
1) An 88 g sample of carbon dioxide is found to contain 24g of carbon and 64g of oxygen. What is the percentage by mass of all elements? Give answers to the nearest whole number.
3) A 57 g sample of sodium carbonate reportedly contains 25g of sodium, 18g of carbon and 20g of oxygen. Do you accept this analysis? Explain why or why not.
5) Pink gold contains 75% gold, 20% copper and 5% silver by mass. Determine the mass of each metal in a pink gold necklace that weighs 20g.
6a) Carbon monoxide is 43% carbon by mass; nitrous oxide is 30% nitrogen by mass. For both gases, the remainder is oxygen. In a mixture containing 5g of each gas, what is the mass of oxygen present?
6b) A 5g sample of carbon monoxide is mixed with 4g of nitrous oxide and 3 g of sulfur dioxide. Sulfur dioxide is 50% sulfur by mass, the balance is oxygen. What is the percentage by mass of oxygen and of sulfur present in the mixture? Use the mass compositions given in part a.
Answers
Carbon: 24 g / 88 g * 100% = 27.3% (rounded to 27%)
Oxygen: 64 g / 88 g * 100% = 72.7% (rounded to 73%)
So, the sample is 27% carbon and 73% oxygen by mass.
To determine whether the reported analysis is correct, we need to check if the masses of the elements add up to the total mass of the sample:
25 g + 18 g + 20 g = 63 g
57 g ≠ 63 g
Since the masses of the elements do not add up to the total mass, the reported analysis is not accepted.
To find the mass of each metal in the necklace, multiply the weight of the necklace by the percentage of each metal:
Gold: 20 g * 75% = 15 g
Copper: 20 g * 20% = 4 g
Silver: 20 g * 5% = 1 g
So, the necklace contains 15 g of gold, 4 g of copper, and 1 g of silver.
6a) To find the mass of oxygen present, subtract the mass of the other two elements from the total mass:
5 g + 5 g = 10 g
Total mass = 10 g
Mass of other elements = 43% * 5 g + 30% * 5 g = 2.15 g + 1.5 g = 3.65 g
Mass of oxygen = 10 g - 3.65 g = 6.35 g
So, there is 6.35 g of oxygen present in the mixture.
6b) To find the percentage by mass of each element, divide the mass of each element by the total mass of the mixture and multiply by 100:
Carbon monoxide: 5 g * 43% = 2.15 g
Nitrous oxide: 4 g * 30% = 1.2 g
Sulfur dioxide: 3 g * 50% = 1.5 g (balance is oxygen: 1.5 g * 100% / 50% = 3 g)
Total mass = 5 g + 4 g + 3 g = 12 g
Mass of other elements = 2.15 g + 1.2 g + 1.5 g + 3 g = 8.85 g
Percentage of oxygen = (12 g - 8.85 g) / 12 g * 100% = 26.25% (rounded to 26%)
Percentage of sulfur = 1.5 g / 12 g * 100% = 12.5%
So, the mixture contains 26% oxygen and 12.5% sulfur by mass
what will be the ph of an aqueous fe(no3)3 solution at 25°c?
Answers
The Ph of an aqueous fe(no3)³ solution at 25°c is totally dissolves all nitrates.
Fe(no3)³ is totally dissolves all nitrates.
In a solution, iron (III) nitrate ionizes to form the following ions:
Fe(No3)³(a q)↔fe²+(a q)+2(No3)-(a q)
Sulfuric acid is a reagent that can be used to differentiate between calcium nitrate and iron (II) nitrate in aqueous solutions. While calcium ions and sulfuric acid combine to form an insoluble precipitate, iron (II) nitrate and sulfuric acid combine to form the soluble iron (II) sulfate ion.
The iron(II) ions and other spectator ions-containing solution is left behind after the calcium ion reacts with sulfuric acid to generate a white precipitate of calcium sulfate. All nitrates are completely soluble in water.
Learn more about aqueous here
https://brainly.com/question/11614686
#SPJ4
Find energy needed to heat 150 g of water from 25. 3 °C to 75. 0 °C with a specific heat of 4. 18 J/g °C.
Answers
Answer: 31161.9 J
Explanation:

Hello how can i get help with science1. State Newton's Third Law of Motion.2. List three examples.3. When you hit a ball, the ball hits back with an equal and opposite force.a. Since the forces are equal and opposite, why does the ball move?b. Why is it that you do not move as far as the ball?
Answers
1. State Newton's Third Law of Motion:
When two bodies interact, they apply forces to one another that are equal in magnitude and opposite in direction. The third law is also known as the law of action and reaction.
2. List three examples:
a. When we jump our legs apply a force to the ground, and the ground applies an equal and opposite reaction and we leave the ground.
b. When we hit the ball with a bat is another example. When the bat hits the ball, the ball goes off the bat with the same force that the bat goes back,
c. When we bounce a ball. A ball is able to bounce because the reaction from the ground. If there was no reaction from the ground, the ball would not bounce. It would stick to the ground.
3. 3. When you hit a ball, the ball hits back with an equal and opposite force.
a. Since the forces are equal and opposite, why does the ball move?
b. Why is it that you do not move as far as the ball?
a. Because the two forces are not applied to the same object. They are two different systems. The ball hits us and makes a force over us. And we hit the ball and produce a force over the ball. But both forces are not applied to the ball, that's why it moves.
b. Because of our weight we are fixed to the ground. We are much heavier than the ball, so our weight is greater than the reaction of the ball.
wel yiald a total of 65,000 gallons of lacquer thinner that can be sold for $9. to a gation. The 2. Should Casidio sol tha acedone as is or process it into laccpor thrreer? 3how the adestional processing will cost 50.60 per gation of lacquer thinner, To sell the lacoguet thinner, Castlla Clyarical must pay shipping of 50.19 a galion and atministrative expenses of 50.13 a gallon on the thinher Requirement 1. Identity the surk cost ta the sunk cost reievant to Casillos decision? Why or why not? Castillo Chemical has spent $242,000 to refine 74,000 gallons of acetone, which can be sold for \$1.90 a gallon. Alternatively, Castillo Chemical can process the acetone further. This processing will yield a total of 65,000 gallons of lacquer thinner that can be sold for $3.10 a gallon. The additional processing will cost $0.60 per gallon of lacquer thinner. To sell the lacquer thinner, Castillo Chemical must pay shipping of $0.19 a gallon and administrative expenses of $0.13 a gallon on the thinner. Requirements 1. Identify the sunk cost. Is the sunk cost relevant to Castillo's decision? Why or why not? 2. Should Castillo sell the acetone as is or process it into lacquer thinner? Show the expected net revenue difference between the two alternatives.
Answers
1. Sunk Cost: The $242,000 spent on refining the acetone is a sunk cost and not relevant to future decisions.
2. Decision Analysis: Processing the acetone into lacquer thinner yields higher expected net revenue ($1,100 more) than selling it as is. Therefore, Castillo Chemical should choose to process the acetone into lacquer thinner.
1. Sunk Cost:
The sunk cost in this scenario is the $242,000 spent on refining the 74,000 gallons of acetone. A sunk cost is a cost that has already been incurred and cannot be recovered, regardless of the decision taken. It is not relevant to Castillo's decision on whether to sell the acetone as is or process it into lacquer thinner. The reason is that the sunk cost is in the past and should not influence future decisions. It cannot be changed or avoided, regardless of the course of action chosen.
2. Decision Analysis:
To determine whether Castillo Chemical should sell the acetone as is or process it into lacquer thinner, we need to compare the expected net revenue from both alternatives.
Option 1: Sell Acetone as is
Revenue from selling 74,000 gallons of acetone at $1.90/gallon = 74,000 gallons * $1.90/gallon = $140,600
Option 2: Process Acetone into Lacquer Thinner
Total revenue from selling 65,000 gallons of lacquer thinner at $3.10/gallon = 65,000 gallons * $3.10/gallon = $201,500
Total Cost of Processing:
Processing cost = 65,000 gallons * $0.60/gallon = $39,000
Shipping cost = 65,000 gallons * $0.19/gallon = $12,350
Administrative expenses = 65,000 gallons * $0.13/gallon = $8,450
Total Cost of Processing = $39,000 + $12,350 + $8,450 = $59,800
Net Revenue Difference:
Net revenue from processing = Total revenue - Total Cost of Processing
Net revenue from processing = $201,500 - $59,800 = $141,700
Expected Net Revenue Difference:
Expected Net Revenue Difference = Net revenue from processing - Revenue from selling acetone as is
Expected Net Revenue Difference = $141,700 - $140,600 = $1,100
The expected net revenue difference between selling the acetone as is and processing it into lacquer thinner is $1,100 in favor of processing the acetone. Therefore, based on the expected net revenue, Castillo Chemical should choose to process the acetone further into lacquer thinner, as it results in higher expected profitability compared to selling the acetone as is.
Learn more about revenue from given link
https://brainly.com/question/29786149
#SPJ11
According to Dalton's Law of Partial Pressures, the pressure of oxygen in dry air would be

Answers
The pressure of the oxygen in the air is 0.21 atm. The partial pressure of a gas is the contribution that gas makes to the total pressure when the gas is part of a mixture.
What is the volume of a nugget of gold that has a mass of 93,300g?
Answers
Answer:
4834.20 ml or 4.8342 L
Explanation:
Gold has density of 19.30 g/ml
so according to d = m/V
V = m/d = 93,300/19.30 = 4834.19689119 = 4834.20 ml or 4.8342 L
The volume of nuggets of gold that has a mass of 93,300g is 4834.20 ml or 4.8342 L.
What is volume?Volume is the space occupied by a three-dimensional object.
Density is the mass per unit volume. Density is a scalar quantity. It is denoted by d and the symbol for density is given as rho, a Greek symbol. Density is calculated as mass divided by volume.
Density is directly proportional to mass and inversely proportional to volume. Thus, with an increase in density, mass increases and volume decreases, and vice-versa
Gold has a density of 19.30 g/ml
so according to density = mass/ volume
V = m/d = 93,300 / 19.30 = 4834.19
4834.20 ml or 4.8342 L
Therefore, the volume of nuggets of gold that has a mass of 93,300g is 4834.20 ml or 4.8342 L.
To learn more about volume, refer to the link:
https://brainly.com/question/13338592
#SPJ2
use the drop-down feature to describe each step of the hydrohalogenation mechanism.
Answers
Here are the steps of the hydrohalogenation mechanism using the drop-down feature:
Step 1: __Initiation__ - A molecule of hydrogen halide (HX) is polarized by a free radical initiator, which breaks the H-X bond, creating a hydrogen atom and a halogen atom.
Step 2: __Propagation__ - The hydrogen atom reacts with the alkene, forming a carbon-centered radical and a molecule of HX. The halogen atom then attacks the carbon-centered radical, forming a new carbon-halogen bond and regenerating the halogen radical.
Step 3: __Termination__ - The reaction continues until all of the alkenes has been consumed or until two free radicals combine, forming a stable molecule.
The hydrohalogenation mechanism can be explained step-by-step using the terms: electrophilic addition, carbocation, nucleophile, and halogen.
1. Electrophilic addition: In hydrohalogenation, an alkene reacts with a hydrogen halide (HX) to form a haloalkane. The reaction starts with electrophilic addition, where the alkene's double bond attracts the electron-deficient hydrogen atom of the hydrogen halide.
2. Carbocation formation: After the alkene's double bond breaks, it forms a carbocation, a positively charged carbon atom. The choice of the carbon where the positive charge resides depends on the stability of the carbocation, with more substituted carbocations being more stable.
3. Nucleophile attack: The halide ion (X-) in the hydrogen halide acts as a nucleophile, attacking the positively charged carbon atom of the carbocation.
4. Halogen addition: The halogen ion bonds to the carbocation, forming a haloalkane product.
Remember that the hydrohalogenation mechanism is an example of electrophilic addition, involving carbocation intermediates and the participation of a nucleophile, which is the halogen ion in this case.
To know more about hydrohalogenation, click below.
https://brainly.com/question/31062019
#SPJ11
if the particles formed through fission have less mass than the starting material has the laaw of conversation of energy been broken
Answers
The law of conservation of energy has not been broken, provided energy is released from the fission process.
What is the law of conservation of energy?The law states that the total energy of a process is conserved. That is, the total energy or mass of a system before and after undergoing processing remains the same. However, some of the mass/energy can be converted to another form.
When a material undergoes fission, the sum total of the mass of the particles formed should be equal to the mass of the starting materials, provided that all other things remain the same.
However, if energy is released from the fission process, it means that some of the mass of the starting materials has been converted to energy and released to the environment.
More on the law of conservation of energy can be found here: https://brainly.com/question/20971995
#SPJ1
Which structure of plant cells helps to maintain homeostasis?
a. semipermeable cell membrane
b. chlorplasts
c. large vacuoles filled with food, air, and water
d. tough, rigid cell wall
Answers
A. Semipermeable call membrane
if 2.75l n2 reacts with 7.75l h2, what is the theoretical yield (in liters) of nh3? assume that the volumes of reactants and products are measured at the same temperature and pressure.
Answers
assuming that the volumes of reactants and products are measured at the same temperature and pressure if 2.75l n2 reacts with 7.75l h2, 10.50 L is the theoretical yield (in liters) of nh3
N2 + 3H2 = 2NH3, volume: 2.75 +7.75 = 10.50. volume of ammonia gas is 10.50 L because of law of conservation of mass Gas volume the correlation among pressure, volume, moles, and temperature. is the volume that mole of a chemical substance or a combination of substances currently takes. As a result, at STP, this same mole ratio volume of a gas is 22.4 litres. A gas's volume refers to the amount of three - dimensional space that the gas occupies. The volume of a gas embedded in a sealed jar is identical to the volume of the container. A system's volume is a critical comprehensive criterion for trying to describe its thermodynamic state. The volume, an intensive estate, is the volume of the system per unit mass.
Learn more about gas volume here:
https://brainly.com/question/15110960
#SPJ4
in a dilution problem, if you are solving for a final concentration (), it should be ____________ than the initial concentration ()
Answers
lower than. The moles per unit volume represent the concentration. Less moles exist in the same volume of the solution when it is diluted, resulting in a lower concentration.
Dilutions. The concentration of an aqueous solution falls when more water is added to it. This is due to the fact that while the total volume of the solution grows, the number of moles of the solute does not.
Dilution is the process in question. The following equation, M1V1 = M2V2, can be used to compare the concentrations and volumes before and after a dilution:
M1 and V1 stand for the molarity and volume of the initial concentrated solution, while M2 and V2 stand for the molarity and volume of the final diluted solution.
To know more about molarity, click the below link
https://brainly.com/question/8732513
#SPJ4
write down the way you had to behave to illustrate the behavior of particles as a gas condenses to form a liquid
Answers
Gases near together and vibrate in position however, don't circulate beyond each other. In a liquid, the particles are interested in every different but now not as a great deal as they may be in a strong.
The particles of a liquid are near together, constantly transferring, and may slide beyond one another. The Kinetic-molecular concept attempts to explain the behavior of fuel molecules based totally on the nature of gasoline. The principle is grounded on simple assumptions
In gases the debris passes swiftly in all directions, regularly colliding with every different facet of the box. With a boom in temperature, the debris gains kinetic strength and passes more quickly. Gasoline is a state of matter that has no constant form and no fixed extent. Gases have a decreased density than other states of the count, together with solids and liquids. there may be a high-quality deal of empty area between debris, that have loads of kinetic energy and aren't especially drawn to one another.
Learn more about the behavior of particles here:-https://brainly.com/question/2456191
#SPJ9
The graph represents the change in that occurs when food is cooked over a charcoal grill. Which statement correctly explains the graph?A. The reactants are unlit charcoal that has already released its energy, and the products are charcoal that has already burned.B. The reactants are charcoal that has already burned and released its energy, and the products are unlit charcoal.C. The reactants are unlit charcoal, and the products are charcoal that has already burned and released its energy.D. The reactants are charcoal that has already burned, and the products are unlit charcoal that has already released its energy.

Answers
The answer is C.
The reactants are charcoal that is unlit + oxygen and the products are the burnt charcoal + energy.
\(C_xH_y+O_{2\text{ }}\rightarrow CO_2+H_2O\text{ + heat}\)So for every combustion reaction like this one, CxHy is the wood. So before you light the wood, it is actally a reactant together with oxygen, because without oxygen the wood will not burn. So under the influence of heat, wood produces substances like carbon dioxide and heat, the moment you see wood burning it it already producing products, CO2 and heat (which is the fire). This is a combustion reaction.
Combustion reaction is exothermic because it releases energy.
As the temperature of a system increases, the entropy _____ due to a(n) _____ in the number of available energy states and thus a(n) _____ in the number of possible arrangements of molecules within those energy states.
Answers
As the temperature of a system increases, the entropy increases due to an increase in the number of available energy states and thus an increase in the number of possible arrangements of molecules within those energy states.
What factors affect Entropy?
As the temperature of a system increases, the entropy increases due to a increase in the number of available energy states and thus an increase in the number of possible arrangements of molecules within those energy states.
This can be understood by the statistical interpretation of entropy, which relates entropy to the number of possible arrangements of molecules in a given energy state. At higher temperatures, the molecules have higher kinetic energy and can occupy a greater number of energy states, resulting in a larger number of possible arrangements of the molecules within those energy states. As a result, the entropy of the system increases with increasing temperature.
To know more about Entropy:
https://brainly.com/question/31066828
#SPJ11
As the temperature of a system increases, the entropy increases due to an increase in the number of available energy states and thus an increase in the number of possible arrangements of molecules within those energy states. This is because at higher temperatures, molecules have more energy and are able to move more freely, increasing the number of ways in which they can arrange themselves within the available energy states.
This increase in entropy is a fundamental principle of thermodynamics, known as the Second Law of Thermodynamics.
What is Second Law of Thermodynamics ?
This law means that in any natural process, some useful energy will inevitably be lost as waste heat, making it unavailable for future use.
In simpler terms, the Second Law states that natural processes always tend towards a state of greater disorder, and it is impossible to convert all of the thermal energy in a system into useful work. This law has important implications for the behavior of engines and the efficiency of energy conversion processes.
To know more about Second Law of Thermodynamics :
https://brainly.com/question/30600157
#SPJ11
Explain how each property affects the energy in a wave:
Amplitude:
Wave speed:
Wavelength:
Frequency:
Answers
Answer:
frequency
Explanation:
because a wave is equally a frequency
Arrange in order of increasing ability to penetrate matter.a. Beta, gamma, alphab. Alpha, gamma, betac. Gamma, beta, alphad. Alpha, beta, gamma
Answers
The correct answer is option b) Alpha, gamma, beta.
Alpha, beta, and gamma are the three main types of ionizing radiation. They differ in their ionizing ability, energy, and penetration power.
Alpha particles have the least penetrating power because they are relatively large and heavy, consisting of two protons and two neutrons bound together. They lose their energy rapidly and can be stopped by a sheet of paper or the outer layer of human skin.
Gamma rays, on the other hand, are highly energetic electromagnetic radiation and have the highest penetrating power. They can easily pass through most materials, including human tissue and lead, and require thick concrete or steel barriers to shield against them.
Beta particles are high-speed electrons emitted by some radioactive isotopes. They have moderate penetrating power and can pass through materials such as plastic and aluminum but are stopped by thicker materials like lead or concrete.
Therefore, the correct order of increasing ability to penetrate matter is alpha, gamma, beta (option b).
For more such questions on Alpha, gamma, beta.
https://brainly.com/question/23426855
#SPJ11
What is the relationship between molecular motion and energy?
Answers
Answer:
Because the particles are in motion, they will have kinetic energy. The particles will not all have the same energy, and the energy of the particles is constantly changing as they undergo changes in speed. Thus, for a given sample of matter, we can only talk about the average kinetic energy of the particles.
Explanation:
I hope this helps and pls mark me brainliest :)
We do know that if we "give" energy to a given object, the temperature of the object increases.
So there is a direct relationship between temperature and energy.
From this idea, we can conclude that:
"As we increase the energy, we also will see an increase in molecular motion."
We also do know that, as the temperature of an object increases, also does the kinetic energy of the particles that conform to the object (as the kinetic energy increases, the particles move more, this is why as we increase the temperature we usually see an increase in the volume of the object).
This increase in kinetic energy is related to the molecular motion (the movement of the electrons and nucleus o a molecule) as the kinetic energy of these parts increase, these move more.
So the relationship between molecular motion and energy is:
As we increase the energy, we also will see an increase in molecular motion.
If you want to learn more, you can read:
https://brainly.com/question/12280673
25. Production is a. Transformation b. Reduction c Consumption d. Valuation
Answers
Answer:
a
Explanation:
I think a is the answer because when you produce something you transform it's raw materials to a finished good.
I hope this helps
What is the main reason plants grow fruit?
Answers
Answer: Fruits contain seeds and develop from the ovaries of flowering plants. The first step in making fruits is pollination. Fruit trees and plants produce flowers. Then, bees, bats, birds, and even the wind spread pollen from one flower to another.
Answer:
Fruits contain seeds and develop from the ovaries of flowering plants. The first step in making fruits is pollination. Fruit trees and plants produce flowers. Then, bees, bats, birds, and even the wind spread pollen from one flower to another.
Explanation:
What is hydrology?
А. the study of weather patterns throughout the earth
B. the study of oceans and freshwater sources
С. the study of the movement and distribution of water on earth
the study of the movement of air throughout the earth

Answers
Answer:
c
Explanation:
Hydrology is the study of the distribution and movement of water both on and below the Earth’s surface, as well as the impact of human activity on water availability and conditions.
I did it on edgy and
A solution conducted an electrical current poorly and turned blue litmus paper red. This solution could be:
Answers
The solution could be an acid. Acids conduct electrical current poorly and turn blue litmus paper red due to their ability to donate protons (H+) to the litmus paper, causing a change in color.
Acids have a pH less than 7 and are known for their sour taste and corrosive properties. They typically contain hydrogen ions (H+) and have a higher concentration of positive ions, making them inefficient conductors of electricity compared to bases or salts. The reaction between the acid and the litmus paper indicates the presence of hydrogen ions, which leads to the color change. Examples of acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). turn blue litmus paper red due to their ability to donate protons (H+) to the litmus paper, causing a change in color.
learn more about Acids here:
https://brainly.com/question/29796621
#SPJ11
True or False: One reason for multiple atomic models over the
years is that atoms and their parts, subatomic particles, are
invisible. Atomic models changed as more technologies
became available to scientists.
A. True
B. False
Answers
Answer:
The answer is A. True
Explanation:
hope i helped
A. True
Atom in simple terms is the basic singular unit into which matter can be divided without any use or discharge of energy.
One of the major reasons for having multiple atomic models over the years is that atom and their parts, subatomic particles, are invisible.
This is where the atomic models came into play. Atomic models were theories about the structure and working of atoms based on the information that was available at that time.
The first atomic model was proposed by J.J Thomson, which is also called the plum pudding model. It proposed that within the atom there were negatively charged particles (electrons) that were floating in a positively charged liquid
The atomic model used today is Schrodinger's atomic model.
learn more about atoms at:
brainly.com/question/28153787
why should the wavelength scale of the spectroscope be calibrated
Answers
To ensure accurate and precise measurements of the wavelengths of light produced by a sample, the wavelength scale of a spectroscope should be calibrated.
The wavelength scale is calibrated by comparing the spectroscope readings to a known standard, such as a calibration lamp that emits light at specific, well-defined wavelengths. This allows the spectroscope to be precisely adjusted so that the correct wavelength for a given sample can be determined.
Failure to calibrate the wavelength scale can result in inaccurate readings and incorrect conclusions about a sample's composition. In analytical spectroscopy, for example, identifying and measuring the specific wavelengths of light emitted by a sample is critical for determining the chemical composition and structure of the sample. Thus, proper calibration is essential to ensure the validity of the results.
To Learn More About wavelength click
https://brainly.com/question/13533093
#SPJ4
How much water is needed to make 7.2moles of glucose?\(6CO2 + 6H2O -\ \textgreater \ C6H12O6 + 6O2\)
Answers
Approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
The balanced equation provided is:
6CO2 + 6H2O -> C6H12O6 + 6O2
From the equation, we can see that for every 6 moles of water (H2O), 1 mole of glucose (C6H12O6) is produced. Therefore, we need to determine the amount of water required to produce 7.2 moles of glucose.
The mole ratio between water and glucose is 6:1. This means that for every 6 moles of water, we obtain 1 mole of glucose. To find the amount of water needed for 7.2 moles of glucose, we set up a proportion using the mole ratio:
(6 moles H2O / 1 mole glucose) = (x moles H2O / 7.2 moles glucose)
Solving for x, we can cross-multiply:
6 moles H2O * 7.2 moles glucose = x moles H2O * 1 mole glucose
43.2 moles H2O = x moles H2O
Therefore, we need 43.2 moles of water to produce 7.2 moles of glucose.
To convert moles of water to grams, we need to know the molar mass of water, which is approximately 18 g/mol. Using the molar mass, we can calculate the mass of water needed:
Mass of water = moles of water * molar mass of water
Mass of water = 43.2 moles * 18 g/mol
Mass of water = 777.6 g
Therefore, approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
for more such question on glucose visit
https://brainly.com/question/397060
#SPJ8