Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia:
N2 (g)+ 3H2(g) → 2NH3(g)
In the second step, ammonia and oxygen react to form nitric acid and water:
NH3(g)+ 2O2(g) → HNO3(g) + H2O(g)
Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest .
Answers
Answer:
-376 kJ.
Explanation:
Related Questions
i just told the guy i like that I like him and he said he wants to get to know me better before making a decision. but now everything feels different. he's been really distant. what does that mean and how do I stop getting so attached?
Answers
Answer: you have to talk to someone who wont mind wanting to wanting to like you a lot like that.
Explanation: I wish I could be able to talk to someone who would want to get to like me like that, so its a very relatable situation.
the elements in period 4 on the peridic table are arranged in order of increasing
Answers
The elements in the 4th period on the periodic table are arranged in order of increasing their atomic number.
The function of elements in the modern periodic table?The modern periodic table can be defined as the arrangement of chemical elements. Elements are orderly arranged according to their increasing atomic number and similar chemical properties. This periodic table is organized in the forms of columns and rows which are named periods and groups.
The 118 elements are present in 18 groups and 7 periods in the modern periodic table. The elements placed in the same group and possessing the same valence electronic configuration possess identical chemical properties. Whereas the elements placed in the same period possess an increasing order of their valence electrons but the electrons are occupied in the same electron shell.
Therefore, in the modern periodic table, the elements are organized as the periodic function of their atomic number. The number of valence electrons is not increased continuously throughout the period of moving left to right.
Learn more about the periodic table, here:
brainly.com/question/11155928
#SPJ1
Which technique can be used to remove air bubbles from a syringe? a) Hold the syringe downward, shake vigorously for 10 seconds, and expel the bubbles through the needle using the syringe plunger. Hold the syringe upright, provide a firm tap or flick to the b) barrel, and expel the bubbles through the needle using the syringe plunger. Hold the syringe upright, shake vigorously for 10 seconds, c) and expel the bubbles through the needle using the syringe plunger. Hold the syringe downward, provide a firm tap or flick to the d) barrel, and expel the bubbles through the needle using the syringe plunger.
Answers
Answer:
Point the syringe upright and tap firmly to move the bubbles to the top and expel by pushing the plunger
Explanation:
If you hold the syringe upside down the bubbles will end up at the bottom of the syringe by the plunger since they float up when tapped
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
g A chemical equilibrium exists when: A chemical equilibrium exists when: there are equal amounts of reactants and products. the rate at which reactants form products is the same as the rate at which products form reactants. the sum of reactant and product concentrations equals one mole. reactants are completely changed to products. the rate at which reactants form products becomes zero.
Answers
Answer:
the rate at which reactants form products is the same as the rate at which products form reactants
Explanation:
There is still a reaction happening just that the second one happens the opposite happens and keeps it at net 0
Use the mole ratio of copper to oxygen rounded off the nearest whole number to
determine both the formula and the molar mass of the compound.
Answers
Formula of the compound is Cu2O and molar mass of the compound is 143.1 g/mol
How to find molar mass of a compound?Make use of chemical formula to determine the number of atoms of each element in the compound.
Multiply atomic weight of each element with its number of atoms present in the compound.
Add up all of them and assign unit as grams/mole.
Determine the molar mass of the compound
Molar mass of the compound can be obtained as followed:
Molar mass of Cu = 63.55 g/mol
Molar mass of O = 16 g/mol
Molar mass of Cu2O = ?
Molar mass of Cu2O = (63.55 × 2) + 16
= 127.1 + 16
= 143.1 g/mol
Learn more about molar masses here:
brainly.com/question/21334167
#SPJ13
for each of these, tell which figure is closest to the correct answer: a) a baseball bat has a length of 100mm or 100 cm or 100 m. b) a glass of milk holds 23 cc or 230 mL, or 23 L. C) a man weighs 75 mg or 75 g or 75 kg. d) a tablespoon contains 15 mL or 150 mL or 1.5 L. e) a paper clip weighs 50 mg or 50 g or 50 kg. F) your hand has a width of 100 mm or 100 cm or 100 m.
Answers
According to the standard metric system, the correct figure indicating the right answer in a is 100 cm of baseball, and in b 230 ml of milk in glass and man of weight 75 kg.
What is standard metric system ?There are certain standard units used for the measurement of each parameter. For example, the mass of a person is said in kg. The volume of a liquid is expressed in ml or L. Similarly, the the width of an area is expressed in mm or cm for a small object.
The length of a the ball is expressed in centimeters. Hence, 100 cm is the answer. The volume of milk in the glass is 230 ml of milk and man of weight 75 kg is correct.
The volume in the table spoon is 15 ml and the weight of paper clip is 50 g. Similarly the width of hand is 100 mm.
Find more on metric system:
https://brainly.com/question/25966695
#SPJ1
What does it depend on ?

Answers
Answer: According to the molecular orbital theory the shape and size of molecular orbital depends upon- Shape and size of combining atomic orbitals.
Explanation:
Which is the best example for genetic diversity?
Answers
Answer:
Genetic Diversity Examples
Different breeds of dogs. ...Different varieties of rose flower, wheat, etc.There are more than 50,000 varieties of rice and more than a thousand varieties of mangoes found in India.Genetic diversity is the total number of genetic characteristics in the genetic makeup of a species, it ranges widely from the number of species to differences within species and can be attributed to the span of survival for a species.
Explanation:
i hope this helps u.
A group of students is investigating how a force applied to an object affects the motion of that object. The diagram shows the setup for the investigation. To create a force, the students add a weight to the weight holder and observe whether the cart moves. The students repeat this step five times, using a different amount of weight each time. Identify the independent and dependent variables of this investigation.
Answers
Answer:
87 is the wight of the hight
Explanation:
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
convert 7.54 x 10^-8 m to nanometers
Answers
7.54 *\(10^8\) meters is 75.4 nanometers.
To convert 7.54 * \(10^8\) meters to nanometers, you can multiply the value by \(10^9\)
as, \(10^9\)nanometers = 1 meter.
7.54 * \(10^8\) m * \(10^9\) = 7.54 x \(10^1\) nm
Therefore, 7.54 *\(10^8\) meters is equal to 75.4 nanometers.
learn more about conversion:
https://brainly.com/question/13076223
To convert 7.54 x 10^-8 meters to nanometers, you multiply 7.54 x 10^-8 by 1 x 10^9 to get 75.4 nanometers.
Explanation:To convert meters to nanometers, you need to know that 1 meter is equivalent to 1 x 109 nanometers. Therefore, if you were to convert 7.54 x 10-8 m to nanometers, you would multiply 7.54 x 10-8 by 1 x 109.
Here's how you'd do it: 7.54 x 10-8 m * 1 x 109 nm/m = 75.4 nm. So, 7.54 x 10-8 meters is equivalent to 75.4 nanometers.
Learn more about Unit Conversion here:https://brainly.com/question/32030244
#SPJ2
what is the relative rate of diffusion of hydrogen and nitrogen
Answers
Answer:
2.645
Explanation:
Rate of diffusion formula:
Sqrt(mass2/mass1)
>>sqrt(14/2)
(Note:Hydrogen must exist in dwiatomic, [H2])
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
Time: Continents Over Time View Hint Place all the images on the timeline before you click the "Check” button. If you get stuck, click on the "View Hint" button, and to start over, click on the "Reset Images" button. C o 225 million years ago 200 million years 135 million years 65 million years ago Today ago ago e Reset images Check Next Chapter: Plates & Boundaries Dunamic Earth Cita Man
Answers
Answer:
Explanation:
45
List three forms of potential energy
Answers
Answer:
Types of Potential Energy
Elastic Potential Energy. Anything that can act like a spring or a rubber band can have elastic potential energy. ...
Gravitational Potential Energy. There is a constant attractive force between the Earth and everything surrounding it, due to gravity. ...
Chemical Potential Energy.
(IF THIS HELPED CAN YOU PLEASE GIVE ME A BRAINYLEST?)
2.75 mol of KClO3 decomposes. How many grams of O2 will be produced?
Answers
As a result, 2.75 moles of KCLO3 are needed.
How do you locate the KClO3 moles?Divide the mass by the molar mass (122.6g/mole) of KClO3 to get the moles, which is 0.626. To determine the amount of O2 produced, multiply that by the molar ratio: (0.062) x (3/2) = 0.939 moles of oxygen.
The balanced chemical equation for KClO3's breakdown is:
2 KClO3(s) → 2 KCl(s) + 3 O2(g)
The equation states that 3 moles of O2 are created for every 2 moles of KClO3 that break down. Thus, we can apply a ratio to determine how much O2 is generated from 2.75 moles of KClO3:
(3 mol O2 / 2 mol KClO3) x 2.75 mol KClO3
= 4.125 mol O2
Now, we may convert moles to grammes using the molar mass of oxygen:
4.125 mol O2 x 32.00 g/mol
= 132 g O2
Therefore, 2.75 moles of KClO3 will produce 132 grams of O2.
To know more about moles visit:-
https://brainly.com/question/26416088
#SPJ1
What is the chemical name of the compound Na2CO3? Use the list of polyatomic ions and the periodic table to help you answer.
A.
sodium carbon oxide
B.
sodium carbonate
C.
sodium(II) carbonate
D.
sodium oxalate
Answers
Answer:
sodium carbonate
Explanation:
sodium carbonate
Janey loves this time of year because she loves eating marshmallow Peeps. One day, she eats ten peeps; 8 of them have a mass of 100 g, 1 of them has a mass of 150 g, and 1 of them has a mass of 200 g. What is the average mass of all the People that Jany ate? Show your work.
Answers
Answer:
115g
Explanation:
100*8=800
800+150+200=1150
1150/10=115
Which of the following can be natural sources of water pollution a volcanic activity be earthquake see algae blooms de all of the above please of the best answer from the choices provided
Answers
Answer:
The correct answer is algae bloom
Explanation:
Algae bloom is a situation in which there is an uncontrollable increase in the amount/growth of algae in a water body (freshwater or marine water). This abnormal increase eventually gives the water body a different colour which is from the pigment of the algae. The processes involved in algae bloom leads to lowered oxygen levels in the water-body which causes the death of several aquatic organisms in the water-body. Since water pollution can be described as the processes involved in causing adverse effects that can contaminate a water-body which can lead to loss of aquatic animals and/or affect the physical properties of the water, algae bloom (as described earlier) is the correct option.
NOTE: volcanic activities and earthquakes may cause air pollution as they release several substances into the air but can hardly be directly linked to water pollution.
Answer:
d) all of the aboved
Explanation:
EDG2020
How many particles are there in 0.057 moles of lithium bromide made
Answers
There are 3.44 x 10^{22} particles in 0.057 moles of lithium bromide.
What chemical compound is lithium bromide known by?The lithium bromide formula also known as the lithium monobromide formula or Bromo lithium formula is explored. It is a counterion bromide-based salt of lithium.
we have to use Avogadro's constant,
Avogadro's constant, is approximately equal to 6.022 x 10^{22} particles per mole.
we can use the following formula:
number of particles = moles x Avogadro's constant
Substitute the values,
number of particles = 0.057 moles x 6.022 x 10^{23} particles/mol
Simplifying the equation
number of particles = 3.44 x 10^{22} particles
To know more about lithium bromide visit:
https://brainly.com/question/16584013
#SPJ9
Name a liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles
Answers
One liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles is hydrochloric acid (HCl). Hydrochloric acid is a strong acid commonly used in laboratories for various purposes, including cleaning and dissolving mineral deposits.
When dry mortar, which is primarily composed of cement, hardens on floor tiles, it can be challenging to remove using traditional cleaning methods. However, hydrochloric acid can effectively dissolve and break down the cementitious components of the mortar.
It is important to note that when using hydrochloric acid, proper safety precautions should be followed, such as wearing protective gloves, goggles, and working in a well-ventilated area.
Additionally, it is crucial to dilute the hydrochloric acid to an appropriate concentration for the specific task, as using it undiluted can cause damage to the tiles or other surfaces.
For more such questions on laboratory
https://brainly.com/question/29455421
#SPJ11
Express 0.00212 in scientific notation.

Answers
Answer:
sis aap ye I'd seach karke follow karo ok plz
Explanation:
baad me unfollow kar dena
XxcutemudaxX
When an atom loses an electron, it gets a _________ charge and is called a ________________. When it gains an electron, it gets a _________charge and is called a ______________ (2)
Answers
Answer:
1. positive 2. Ion 3. Negative 4. anion
Explanation:
Hope this helps you! :)
Convert 6598 mL to cubic centimeters (1 mL=1cm^3
Answers
Answer:
6598 mL = 6598 cm^3
Explanation:
Cubic centimeters (cc) and milliliters (mL) are both units of volume measurement. They are equivalent, meaning that 1 mL is equal to 1 cm^3. To convert from mL to cm^3, we simply multiply the volume in mL by 1.
To convert 6598 mL to cubic centimeters, we can use this conversion factor:
1 mL = 1 cm^3
Therefore, we can multiply 6598 mL by 1 to get the equivalent volume in cubic centimeters.
6598 mL x 1 cm^3/mL = 6598 cm^3
So, 6598 mL is equal to 6598 cm^3.
What is the new concentration? L
M NaCl
Answers
Answer:
Explanation:
\we must convert the mass of NaCl in grams into moles. We do this by dividing by the molecular weight of NaCl (58.4 g/mole). Then, we divide the number of moles by the total solution volume to get concentration. The NaCl solution is a 0.1 M solution.
Answer:
0.125
Explanation:
boom
c. A 75 lb (34 kg) boy falls out of a tree from a height of 10 ft (3 m). (3 points)
i. What is the kinetic energy of the boy when he hits the ground? Round your answer to the nearest joule. (1 point)
ii. What is the speed of the boy when he hits the ground? Round your answer to two significant figures. (1 point)
iii. Using the conversion factors of 1 m = 1.094 yd and 1 mi = 1760 yd, calculate the speed of the boy in miles per hour when he hits the ground. (1 point)
Answers
(I) kinetic energy of the boy when he hits the ground =potential energy
I. e ½mv²=mgh where g is 10ms-²
m=34kg, g=10ms² , h=3m
=34×10×3
=1020j
(i i) v²=u²+2gh
v²=0²+2×10×3
= 60
v=sqrt of 60
What was the theoretical yield of a reaction if 50g are formed with a 10% yield?
A: 200g
B: 100g
C: 250g
D: 500g
Answers
Answer: 500g
Explanation:
We are asked to find the theoretical yield of a reaction, and we are given the following information:
In order to find the the theoretical yield we must use the following formula:
We have to convert the percents to real numbers before the calculations. We can do it dividing the percent value into 100, so:
Step 4: Summarize nuclear changes.
a) Use your completed table and models to write a summary that addresses the following questions:
1. How does energy change in these reactions? Is energy needed to start the reactions or is energy given off in the reactions? For each type of reaction, approximately how much
energy is released?
2. How do these energy changes compare in scale to other types of reactions, such as chemical reactions?
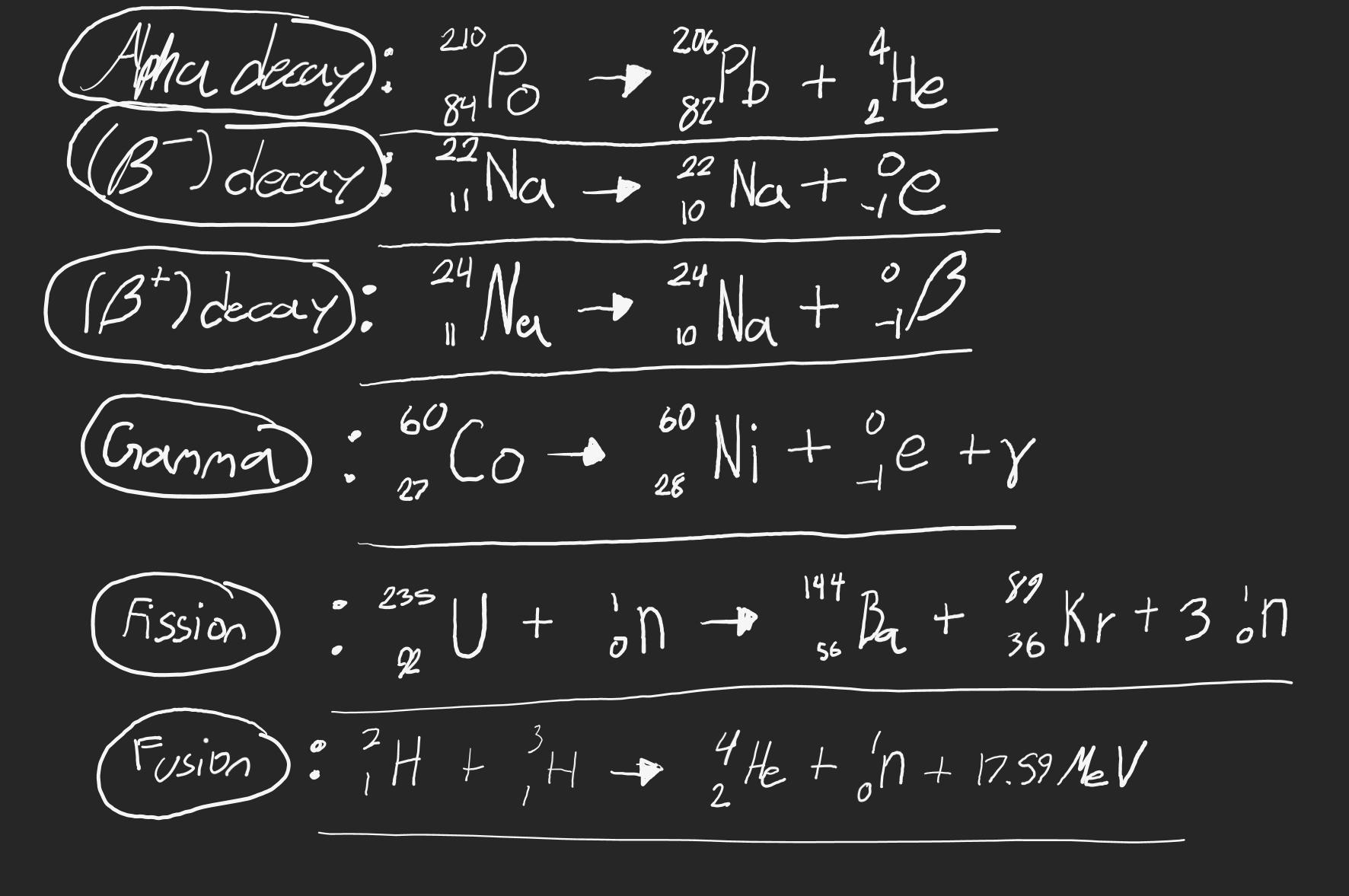
Answers
Nuclear changes involves changes in the composition of the nucleus of atoms with the release of large amounts of energy.
What are nuclear changes?Nuclear changes are changes which occur in the nucleus of atoms of elements.
Nuclear changes involves radioactive decays to give radiation and energy spontaneously known as radioactivity.
In this type of nuclear change no energy is required to start the reaction but large amount of energy are released.
Nuclear changes can also involve splitting of large nucleus into smaller nucleus of atoms known as nuclear fission or the formation of larger nucleus from the combination of two or more smaller nuclei known as nuclear fusion.
Energy is required to start nuclear fission and fusion but the energy released is far greater.
When compared with energy changes in chemical reactions, the energy released in nuclear reactions is far greater.
Therefore, nuclear changes involves changes in the composition of the nucleus of atoms with the release of large amounts of energy.
Learn more about nuclear changes at: https://brainly.com/question/25819143
#SPJ1
the human population grew form 1 billion in the year 1800to blank billion in the year 200
Answers
The human population grew from 1 billion in the year 1800 to approximately 7.8 billion in the year 2021.
In the year 1800, the estimated global human population was around 1 billion. Over the next two centuries, significant advancements in technology, medicine, agriculture, and improved living conditions contributed to a rapid increase in population.
The growth rate of the human population began to accelerate in the 20th century. By the year 1927, the global population reached 2 billion. It took just 33 years for the population to double, reaching 4 billion in 1960. The population continued to grow at an unprecedented rate, with 6 billion people on Earth by the year 1999. As of 2021, the estimated global population stands at approximately 7.8 billion.
This remarkable growth in population can be attributed to several factors, including advancements in healthcare leading to reduced infant mortality rates, improved access to education and contraception, increased agricultural productivity, and overall socio-economic development.
It's important to note that population growth has not been uniform across all regions. Different countries and regions have experienced varying rates of population growth due to factors such as fertility rates, mortality rates, migration patterns, and government policies.
For more such questions on population visit:
https://brainly.com/question/30148263
#SPJ8